Atomic Structure and Symbolism (Needs Math)
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
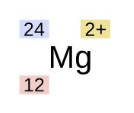
This is the format for isotopes/ions. Name the colors based on what they are:
Blue: Atomic mass (P + Neutrons)
Red: Atomic number (protons)
Yellow: Charge (protons - electrons)
1 amu is equal to ___ the mass of one ___ atom. This is used because oxygen-16 weighs about 16/12 times heavier than carbon-12, so oxygen is ___ amu
1/12, carbon-12, 16
Because atoms can exist naturally as two or more isotopes, the average atomic mass is calculated by what?
(% abundance x isotope mass)
19.9% of ^10B is 10.0129 amu, and 80.1% of 11^B is 11.0093 amu. Find average mass of boron:
(% abundance x isotope mass) + (% abundance x isotope mass)
Two isotopes of boron: ^10B and ^11B, has masses of 10.0129 and 11.0093 amu, and the average atomic mass of boron is 10.81 amu. How do you find % of each isotope?
10.0129x + 11.0093y = 10.81, then use method of substitution.
Similarities between isotopes: They have the same number of ___, element ___, and similar chemical ___.
Differences: Number of ___ are different.
protons, identity, behavior
neutrons
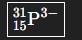
How would you write the ion with atomic number 15, mass number 31, and 3- charge?
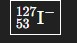
How would you write the ion with 54 electrons, 53 protons, and 74 neutrons?