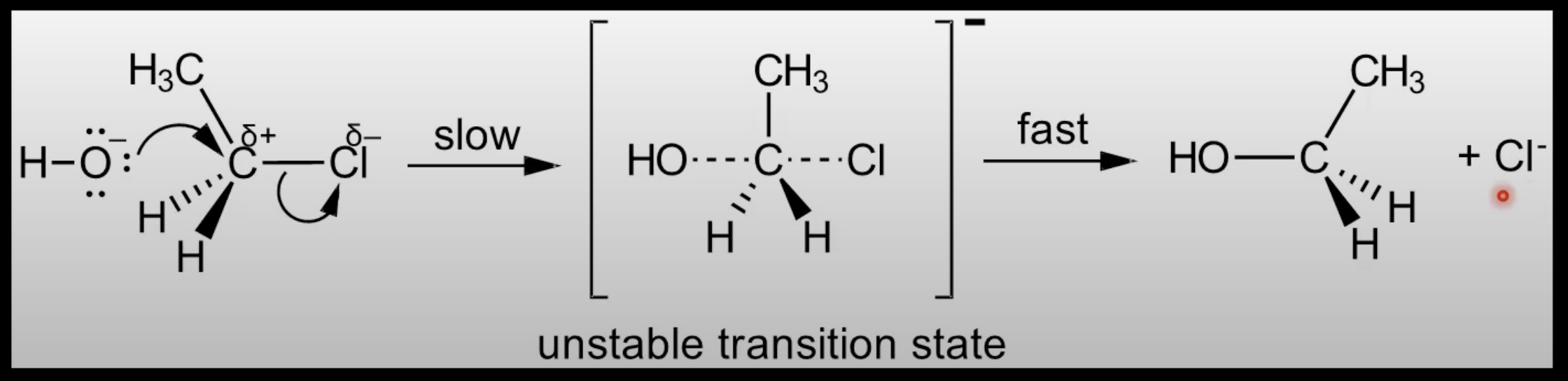
R3.3.1/R3.4.3 Heterolytic/homolytic bond fission
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
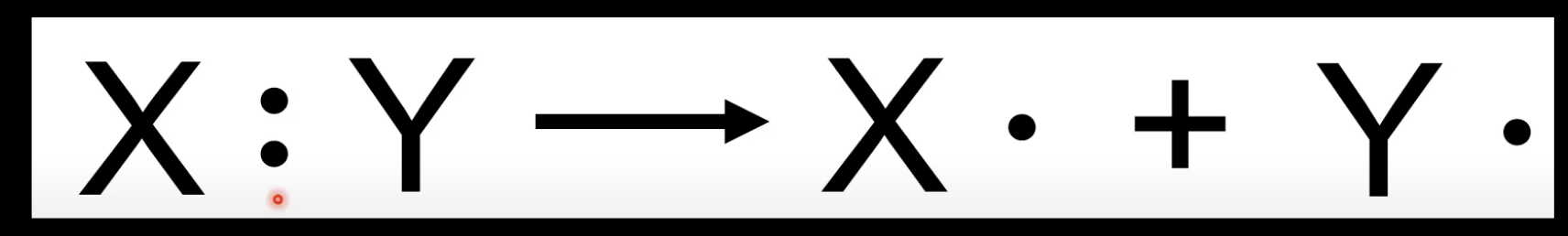
Homolytic bond fission
Covalent bond breaks evenly
Each atom takes one electron.
Forms free radicals with unpaired electrons.
Represented with a dot (•).
2
New cards
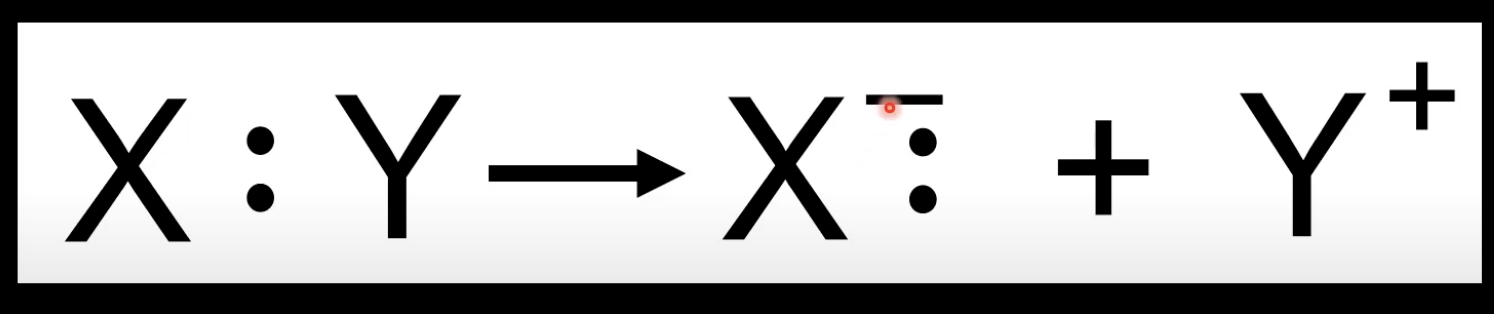
Heterolytic bond fission
Covalent bond breaks unevenly
One atom takes both electrons.
Forms positive and negative ions.
More electronegative atom gets both electrons.
3
New cards
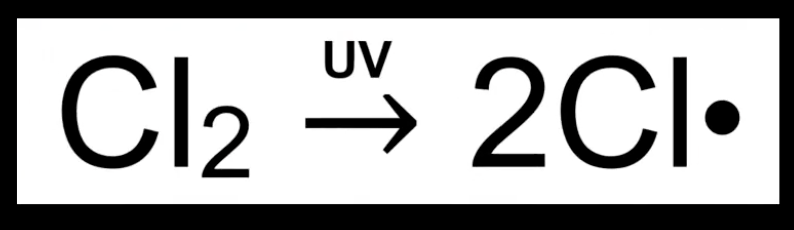
Homolytic fission example
Cl–Cl bond breaks under UV light
Each Cl takes one electron.
Produces two Cl• radicals.
Seen in free radical substitution.
4
New cards
Heterolytic fission example
C–Cl bond in haloalkane breaks unevenly.
Cl gets both electrons forming Cl⁻.
Seen in nucleophilic substitution.