Chem II Ch. 16, 17, 18, 19
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
Qsp < Ksp
No precipitate
Qsp = Ksp
No precipitate
Qsp > Ksp
Yes precipitate
Common Ion Effect
Influence (usually a decrease in solubility) an ion already in solution has on the solubility of an ionic compound
Usually decreases solubility if the acid shares a common ion with the salt
Solving For Molar Solubility w/ Common Ion Effect
Identify the common ion between the two compounds
the given concentration of the other compound (not the one you find Ksp for) will be the concentration of the common ion
Write rxn of main compound breaking down into its ions
Make RICE table (main compound = solid) & put concentration of common ion
if main compound has OH, always assume the OH came from H2O ( [OH] from H2O = 1 × 10-7 M)
Find Ksp and plug in equilibrium expression to find s
Metal hydroxides & compounds with strong or weak base anions have …
Higher solubility in acidic solutions
Mg/Ca/Sr/Ba with (OH)2 have …
Lower solubility in basic solutions because of the common ion effect
Most other metal hydroxides have …
Increased solubility in basic solutions due to the formation of a complex ion
Salts that contain a basic anion are …
More soluble in strong acids and the anion will react with H+ (aq) to form its weak conjugate acid
Steps for Expecting Solubility to be More, Less, or Same as in Pure Water Solution
Write what ions each salt breaks into when dissolved in H2O
a) If the salt shares a common ion with the acid (common ion effect), solubility will be less than in pure water solution
b) If above doesn’t apply, continue to Step 2
Write eqn of salt + strong acid
if it forms a weak conjugate acid, its solubility is more than in pure water solution
Selective Precipitation Definition
Using differences in solubility to separate mixtures of ions
Steps for Determining Concentration and Whether There is Precipitation
Ignoring neutral ions, write out what precipitate is formed when combining solutions
Ex: NaCO3 + MgNO3: MgCO3 (s) → Mg2+ (aq) + CO32- (aq)
Write out Ksp eqn and find its table value
You need to compare Q with Ksp, but need to find Q first
Calculate concentration of both ions, plug them into Q, and compare with Ksp
Qsp > Ksp = a precipitate forms
Qsp < Ksp = no precipitate forms
Create RICE Table
Solve for concentration of each ions as normal
1st Law of Thermodynamics
1) the energy of the universe is conserved (constant)
2) the energy of an isolated system is conserved
3) the change in internal energy for a closed system is equal to the heat and work exchanged
Entropy, S - Information Theory Perspective
Allows us to calculate the absolute entropy of a system
2nd Law of Thermodynamics
A spontaneous process in an isolated system always increases the entropy of the system
Entropy, S General Definition
A measure of how much you don’t know about a macrostate
Factors that Increase Entropy
Increased Volume of gas at constant temp
More Matter on Product Side of Rxn
Increased Temperature
Ssolid < Sliquid < Sgas
3rd Law of Thermodynamics
The entropy of a perfect crystal at 0 K is zero
Entropy - Thermodynamic Perspective
The change in entropy is the reversible heat exchanged at temperature, T
Formula for Finding △S°universe
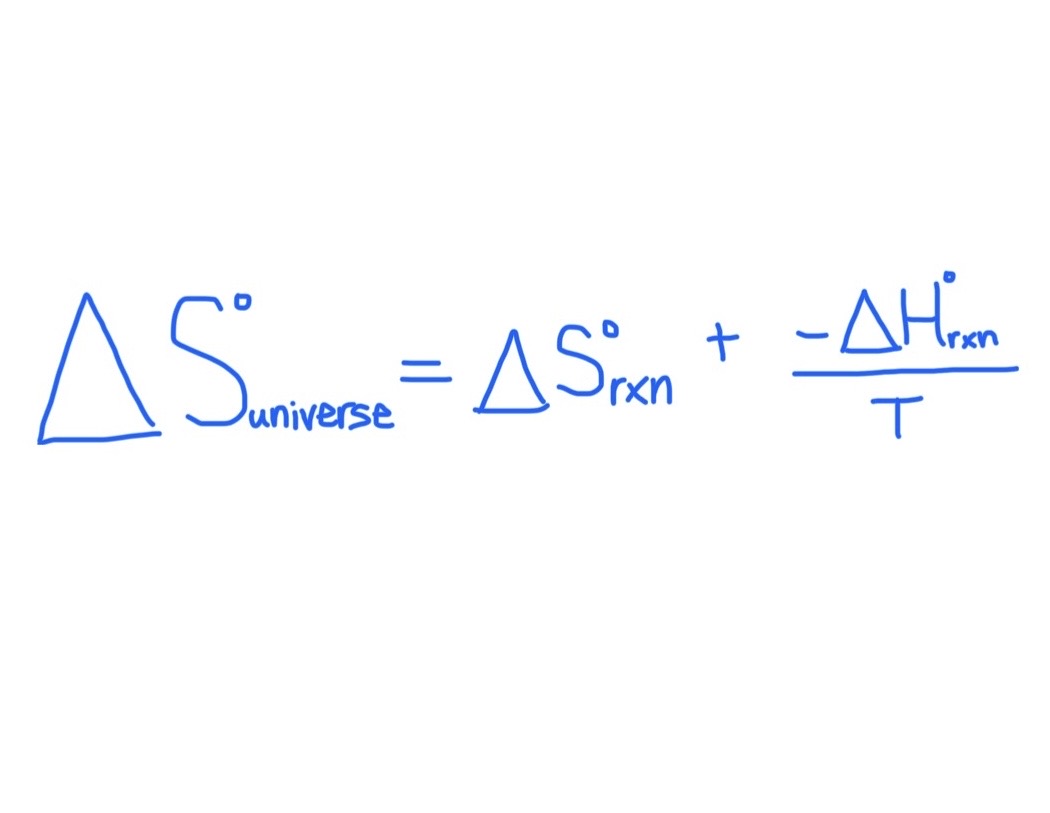
If △S°universe is positive …
Then is product favored aka thermodynamically favored to occur
If △S°universe is negative …
Then is not product favored
At low temp …
△H°rxn has the most effect on sign of △S°universe
At high temp …
△S°rxn has the most effect on sign of △S°universe
Reversible
1) forward & backward change follows the same path
2) equations of state are always obeyed
3) the reversible path as infinitesimally small steps so that the system & surroundiings are always in equilibrium
Formula for Finding △S°rxn
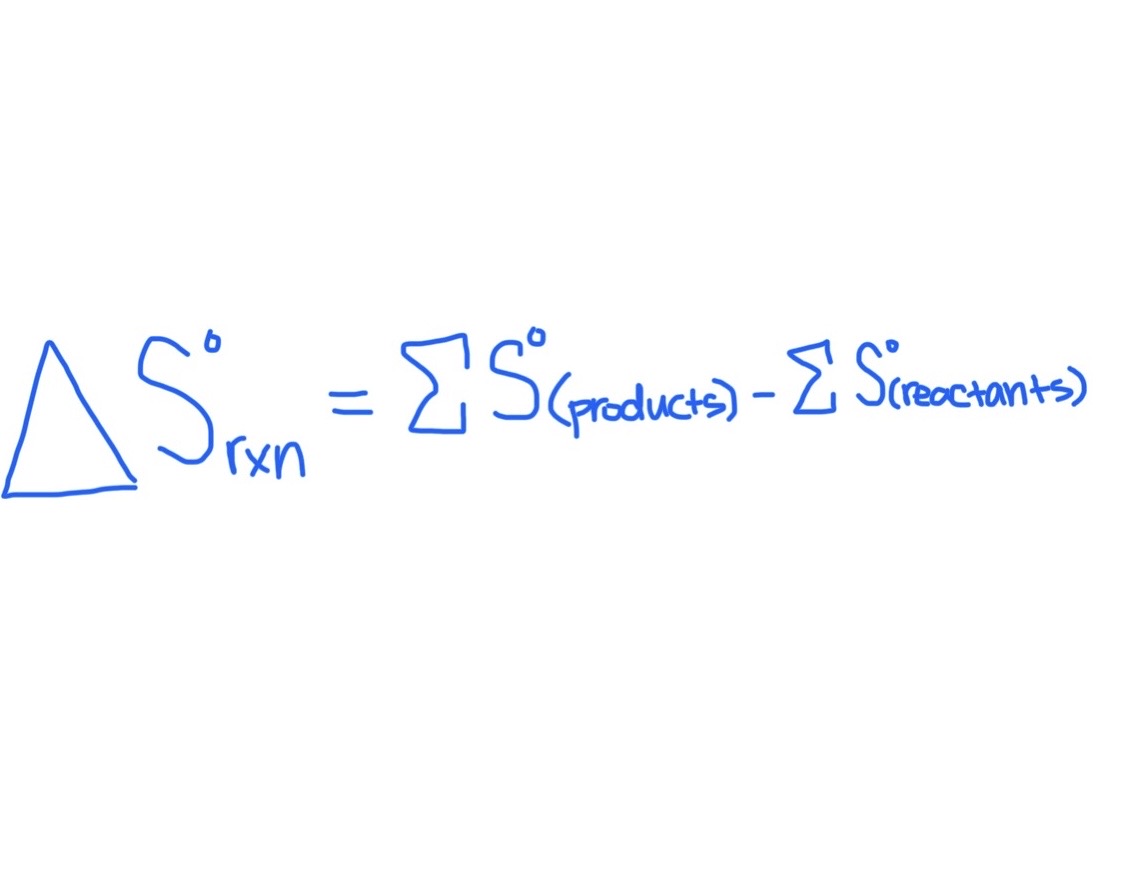
Factors in Determining Entropy
1) Phase
Ssolid < Sliquid < Sgas
2) Molar Mass
Heavier = higher entropy
3) Molecular Complexity
more complex = higher entropy
Formula for Finding △H°rxn
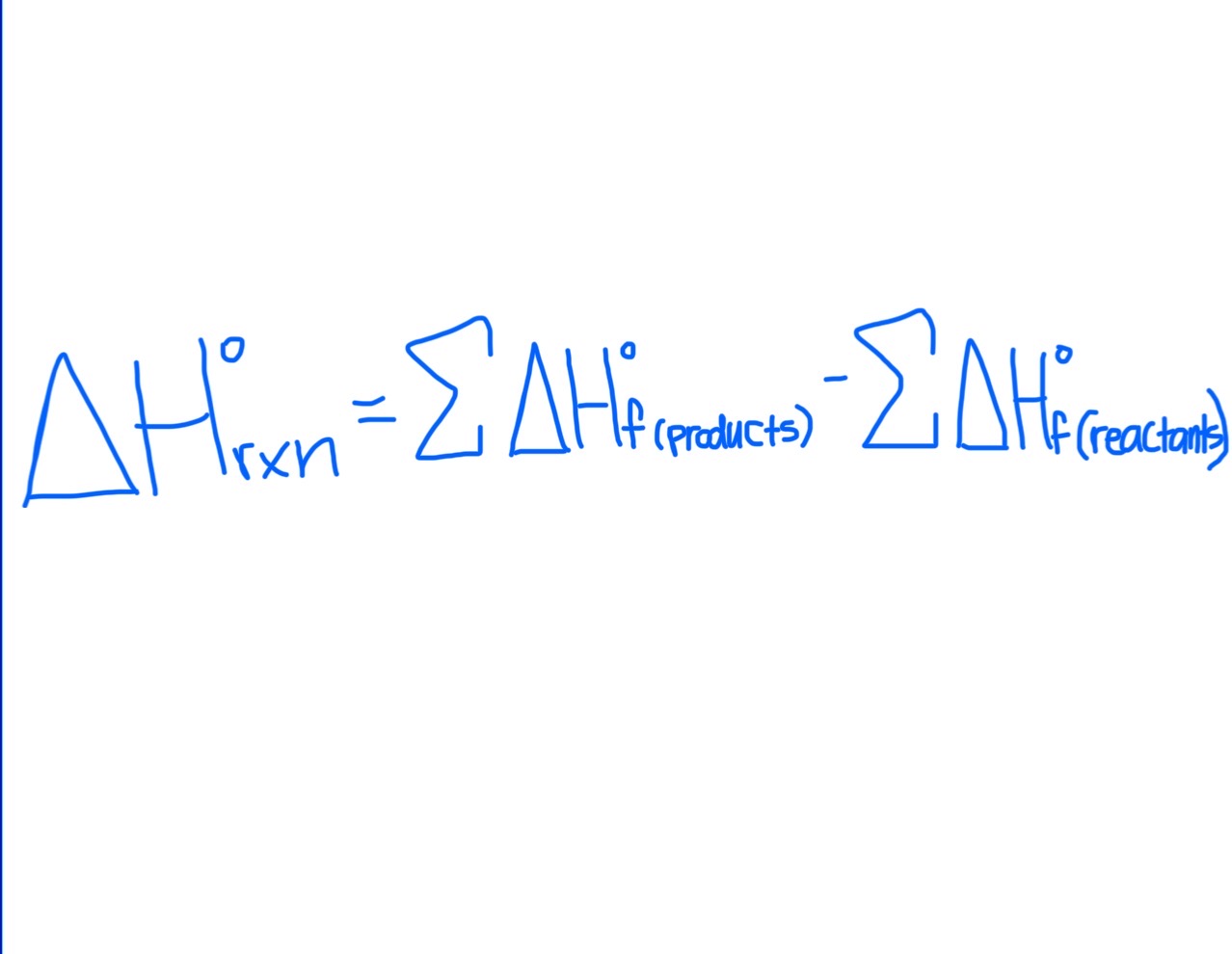
Formula for Finding △Ssurroundings
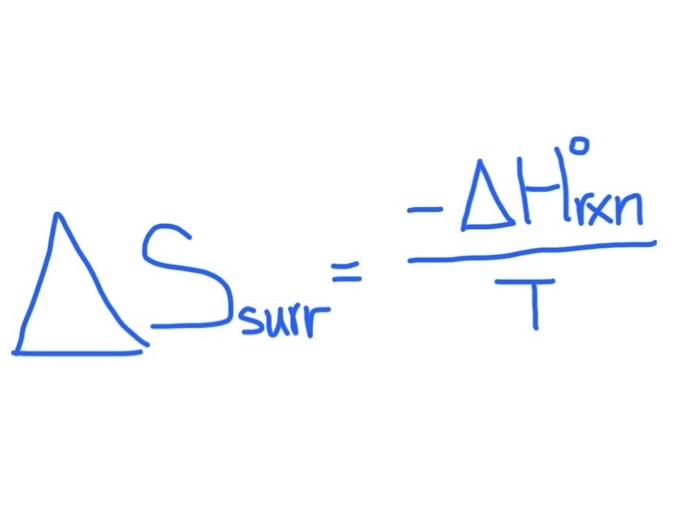
If Endothermic Rxn, Entropy of Surroundings …
Decreases
If Exothermic Rxn, Entropy of Surroundings …
Increases
When △Suniv > 0 and △G°rxn < 0 …
Spontaneous, K > 1; product favored
When △Suniv < 0 and △G°rxn > 0 …
Non spontaneous, K < 1; reactant favored
When △S°rxn = + and △H°rxn = + …
Spontaneous at High Temp
When △S°rxn = - and △H°rxn = - …
Spontaneous at Low Temp
When △S°rxn = + and △H°rxn = - …
Spontaneous at All Temp
When △S°rxn = - and △H°rxn = + …
Spontaneous at No Temp
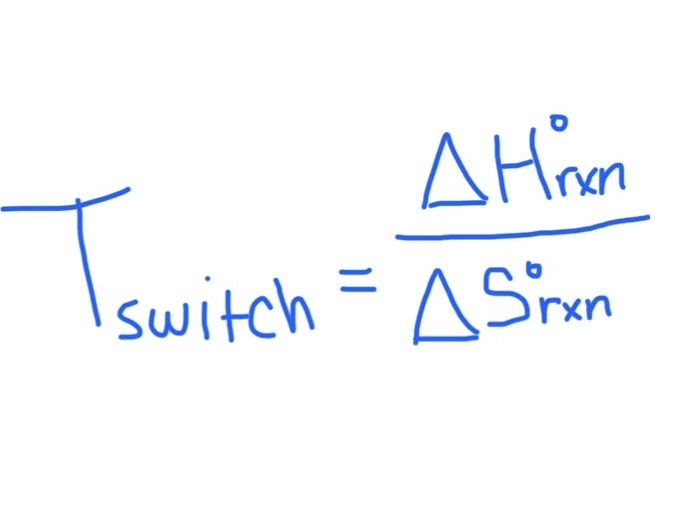
Formula for Finding Tswitch
Formula for Finding △G°rxn
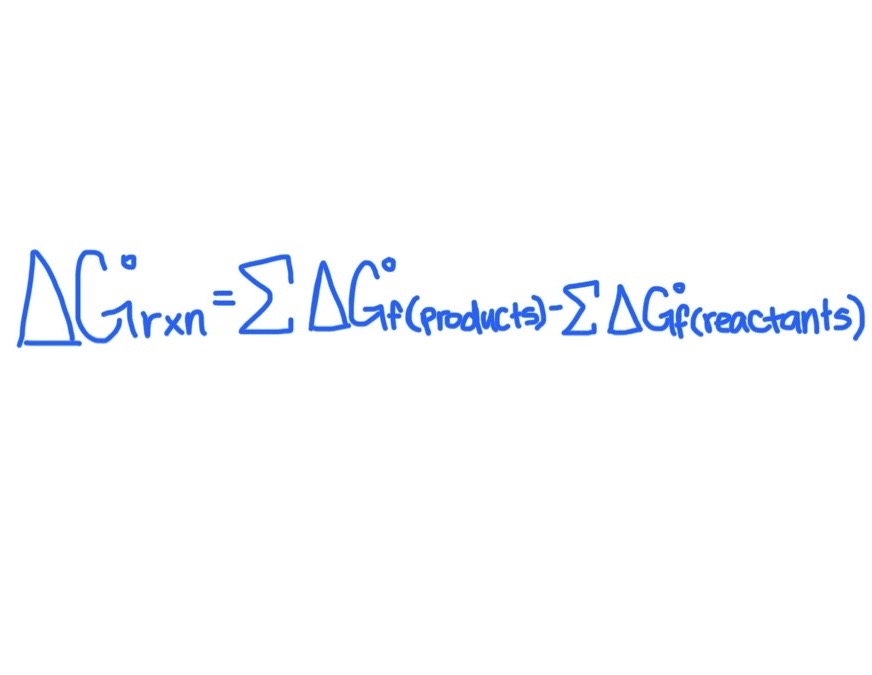
△G°rxn Definition
Change in free energy to create 1 mol of compound from its elements in their standard state
If Reverse a Rxn …
Flip sign of △G°rxn
If multiply a rxn by a factor …
Multiply △G°rxn by the factor
If add two rxns …
Add △G°rxn together
Formula for Finding △G
R = 8.314 × 10-3
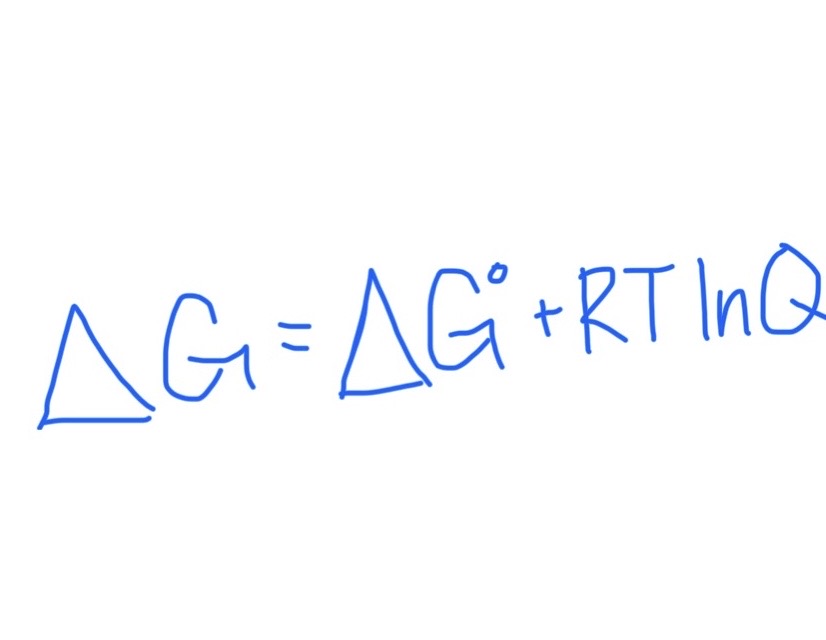
The better the agent …
the worse the conjugate
Balancing Redox Rxns in Pure H2O
Look at the main chemical rxn and identify what is oxidized and reduced
Write out the oxidation half rxn and write out how many electrons is added and on which side of rxn
Write out the reduction half rxn and do same thing
Combine them back
Balancing Redox Rxns in Acidic Solutions
Balance the electrons of the non-oxygen / central atoms
Balance O atoms with H2O by adding however H2O on the other side to satisfy the amount of needed O atoms
Balance H atoms with H+ by looking adding however H+ atoms on other side to satisfy the amount of needed H atoms
Balance charge of e- by adding necessary electrons on either side
Balancing Redox Rxns in Basic Conditions
Balance central atoms
Balance O atoms with OH-
Balance H atoms with H+
Add OH- to both sides to turn H+ and OH- into H2O
Balance charge with e-
Oxidation is always on …
The left
Reduction is always on …
The right
Reference Cell
Standard hydrogen electrode (SHE)
H2 (g, 1 atm)
Formula for Finding E°cell
E°cathode - E°anode
If E°cell > 0 and △G < 0
Spontaneous in the direction written, Q < K
If E°cell < 0 and △G > 0
Non spontaneous, Q < K, backward rxn
Candidates for Reduction
The more positive reduction potential
Candidates for What Gets Oxidized
The more negative reduction potential
If Atomic # > amu
Have too many neutrons, use β- - decay to get rid of a neutron
If Atomic # < amu
Don’t have enough neutrons, so use β+ (positron emission) or electron capture to make a neutron
If both heavy AND too few neutrons
Use α - decay
S° > 0
Have more mol of gas on product side
Product Favored
S° < 0
Have more mol of gas on reactant side
Reactant favored
s → l
l → g
g → s
△S > 0, spontaneous
l → s
g→ l
△S < 0, nonspontaneous
Formula for Finding Total Charge Passed (in coulombs)
Q = I * t
I = the current in amperes (C/s)
t = time in seconds
Formula for Converting Charge to Moles of Electrons
mol e- = Q/F
F = faraday’s constant
Steps for Finding Amount of Grams Able to be Electroplated
Use Q = I*t to find total charge passed (answer will be in C units)
Convert charge to mole e- by using mol e- = Q/F
Write down half reaction including electrons added
Divide answer from Step 2 by the amount of electrons
Convert mol to grams
If Asked What Will Reduce H+ Spontaneously
Look at table with standard reduction potentials
metals above hydrogen
If Asked to Find Change in Gibbs free energy
Use △G = -nFEcell
n = number of electrons transferred
F = faraday’s constant
Ecell = Ecathode - Eanode
Good Candidates for being a Reducing Agent
More negative standard reduction potential
Good Candidates for being an Oxidizing Agent
Less negative standard reduction potential
Use Nernst Equation When …
The electrochemical cell is not at standard conditions
not at 1 M, 1 atm, or 298 K
Equation for Q in lnQ
[products / [reactants]
Form of electrochemical cell notation
reactant | product || reactant | product
The salt bridge completes the circuit by allowing __ to flow towards the anode cell and __ to flow towards the cathode cell
Anions, cations
In Chemical Rxn, what can be oxidized and reduced/ oxidizing and reducing agents?
Only species on the reactant side
For alpha decay …
Mass # decreases by 4, atomic # decreases by 2
For beta- decay …
Mass # stays the same, atomic # increases
For positron emission (beta+) and electron capture …
Mass # stays the same, atomic # decreases
in Ebinding = -△mc2 / A
△m = actual mass - sum of subatomic particle mass
Steps for Solving for Ebinding per nucleon
Determine the number of protons and neutrons
M - A = N
Multiply the number of protons and neutrons to the mass numbers given accordingly
Find △m (actual mass - sum of subatomic particle mass) and convert to kg/mol by adding x 10-3 to the end of the number
Plug numbers into equation where A = atomic number