Chemistry Basics!
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
Which elements are diatomic?
The diatomic elements are Hydrogen (H2), Nitrogen (N2), Oxygen (O2), Fluorine (F2), Chlorine (Cl2), Bromine (Br2), and Iodine (I2).
What are the six strong acids?
1. Hydrochloric acid (HCl) 2. Hydrobromic acid (HBr) 3. Hydroiodic acid (HI) 4. Nitric acid (HNO3) 5. Sulfuric acid (H2SO4) 6. Perchloric acid (HClO4) 7. Chloric acid (HClO3) is sometimes included.
What are the only two liquid elements at 25°C?
Bromine (Br) and Mercury (Hg).
What are the five classes of compounds in chemistry?
1. Molecular 2. Ionic 3. Acids 4. Complex ions 5. Organic.
What is an example of a molecular compound?
Ammonia (NH3) is an example of a molecular compound.
What type of compound is formed if it starts with a metal or ammonium?
Ionic compound.
What type of compound is formed if it starts with a nonmetal or metalloid?
Molecular compound, which may be an acid or organic.
What is the general rule for identifying an acid based on its formula?
If it starts with hydrogen, it's probably an acid.
What is the naming convention for molecular compounds?
Compounds end in -ide and use prefixes to indicate the number of atoms.
What is the prefix for the number 2 in molecular compound naming?
Di-.
What is the prefix for the number 3 in molecular compound naming?
Tri-.
What is the prefix for the number 4 in molecular compound naming?
Tetra-.
What is the prefix for the number 5 in molecular compound naming?
Penta-.
What is the prefix for the number 6 in molecular compound naming?
Hexa-.
What is the prefix for the number 7 in molecular compound naming?
Hepta-.
What is the prefix for the number 8 in molecular compound naming?
Octa-.
What is the prefix for the number 9 in molecular compound naming?
Nona-.
What is the prefix for the number 10 in molecular compound naming?
Deca-.
What are ionic compounds formed from?
A metal or ammonium (positive cation) and a nonmetal (negative anion) or negative polyatomic ion.
How do metals typically form cations?
By losing electrons.
How do nonmetals typically form anions?
By gaining electrons.
What is the process for naming anions?
Drop the ending of the element name and add -ide (e.g., Cl becomes chloride).
What charge do elements in column 5 typically have when forming ions?
They typically gain 3 electrons to achieve an octet, resulting in a -3 charge (e.g., P-3, N-3, As-3).
What charge do elements in column 6 typically have when forming ions?
They gain 2 electrons to achieve an octet, resulting in a -2 charge (e.g., O-2, S-2, Se-2, Te-2).
What charge do halogens in column 7 typically have when forming ions?
They gain 1 electron to achieve an octet, resulting in a -1 charge (e.g., F-1, Cl-1, Br-1, I-1).
What is the formula for the ionic compound formed from Ca+2 and P-3?
Ca3P2.
What is the formula for the ionic compound formed from Al+3 and O-2?
Al2O3.
What is the formula for the ionic compound formed from Al+3 and Cl-1?
AlCl3.
What is the formula for the ionic compound formed from Na+1 and P-3?
Na3P.
What is the formula for the ionic compound formed from Na+1 and Cl-1?
NaCl.
What is the formula for the ionic compound formed from Ca+2 and Cl-1?
CaCl2.
What is the formula for the ionic compound formed from Na+1 and O-2?
Na2O.
What must you do when dealing with transition metals regarding their charges?
You must be given a roman numeral in the name to know the charge on the metal.
What is the charge of Iron(III) in Iron(III)sulfide?
Fe+3.
Which metals do not require a roman numeral in their names?
Silver (Ag), Cadmium (Cd), and Zinc (Zn) always have the same charge (Ag+1, Cd+2, Zn+2).
What is the formula for the compound formed from Copper (I) and Sulfur?
Cu2S.
What is the formula for the compound formed from Lead (IV) and Nitrogen?
Pb3N4.
What is the formula for the compound formed from Iron (II) and Bromine?
FeBr2.
What is a polyatomic ion?
A group of atoms that carry a charge.
What is the formula for the compound formed from Ca+2 and CO3-2?
CaCO3.
What is the formula for the compound formed from Al+3 and SO4-2?
Al2(SO4)3.
What is the formula for the compound formed from Ga+3 and NO3-1?
Ga(NO3)3.
What is the formula for the carbonate ion?
CO3-2
What is the formula for the sulfate ion?
SO4-2
What is the formula for the phosphate ion?
PO4-3
What is the formula for the ammonium ion?
NH4+1
What is the formula for the nitrate ion?
NO3-1
What is the charge of sodium ion?
Na+1
What is the formula for calcium phosphate?
Ca3(PO4)2
What is the charge of zinc ion?
Zn+2
What is the charge of iron(III) ion?
Fe+3
What is the charge of cobalt(III) ion?
Co+3
What is the naming convention for binary ionic compounds?
They end in -ide.
How do you name ZnI2?
Zinc iodide.
What is the name of Na2CO3?
Sodium carbonate.
What is the name of BeO?
Beryllium oxide.
What is the name of CCl4?
Carbon tetrachloride.
How do you name N2O?
Dinitrogen monoxide.
What is the name of Au3PO3?
Gold(III) phosphate.
How do you name Pt3P2?
Platinum(II) phosphide.
What is the name of AgNO3?
Silver nitrate.
What is the name of Ca3(PO4)2?
Calcium phosphate.
What is the formula for magnesium hydroxide tetrahydrate?
Mg(OH)2•4H2O.
How do you convert Celsius to Kelvin?
K = ºC + 273.
What is the scientific notation for 0.000985?
9.85 x 10^-4.
Why does it not matter if the temperature change is recorded in Celsius or Kelvin?
Both scales have the same incremental value; a change of 1°C is equal to a change of 1 K.
What is an ion?
An atom or molecule that has a positive or negative charge due to an imbalance of protons and electrons.
What determines the atomic number of an atom?
The atomic number is determined by the number of protons in the nucleus.
What is the mass number of an atom?
The mass number is the total number of protons and neutrons in the nucleus.
What are isotopes?
Isotopes are atoms of the same element that have different numbers of neutrons and therefore different mass numbers.
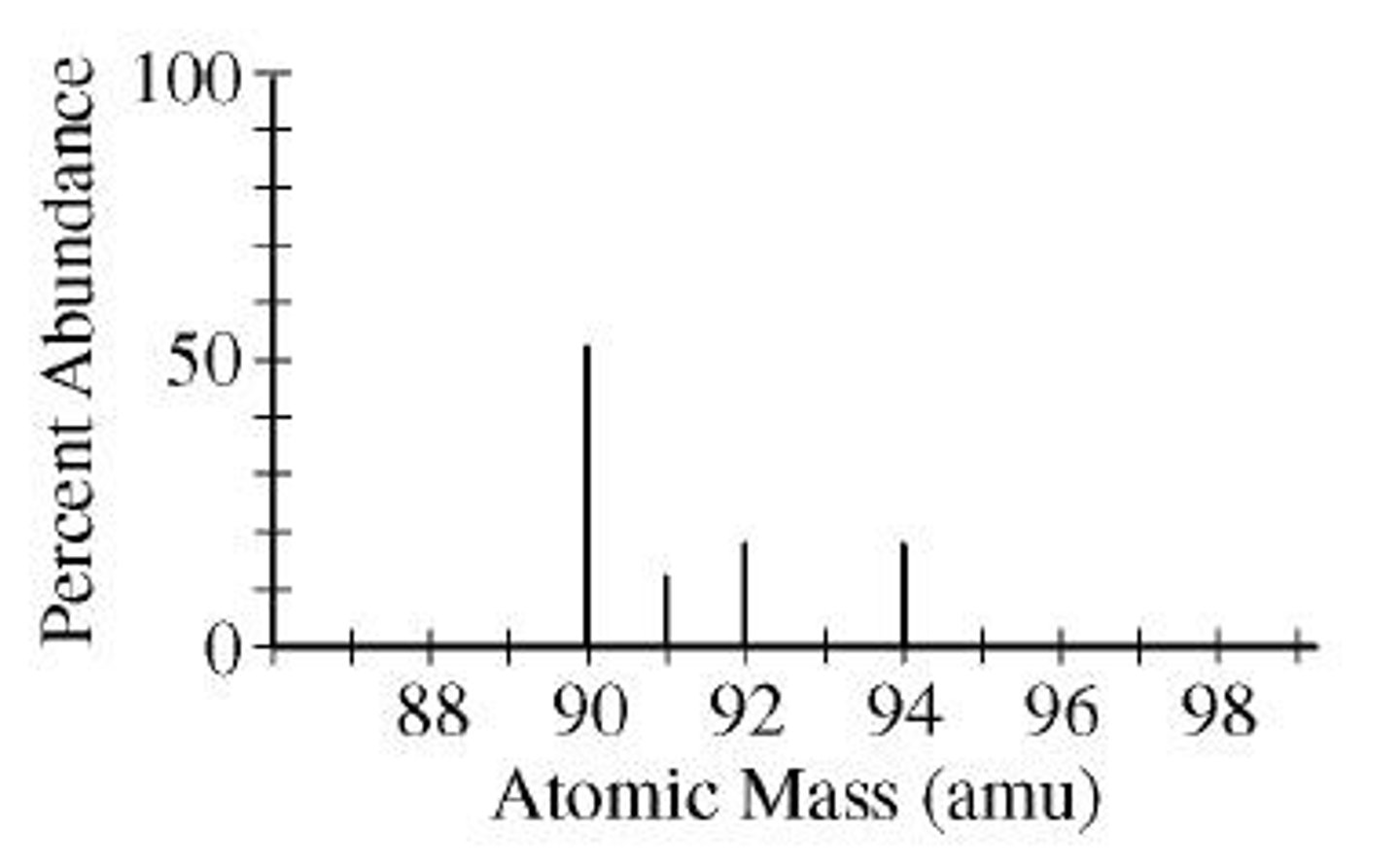
How is an isotope described?
An isotope is described by its mass number, such as carbon-12 or carbon-14.
What is a chemical symbol?
A shorthand notation that includes the element symbol, atomic number, mass number, and charge.
How is shorthand notation for an ion represented?
The shorthand notation includes the element symbol in the center, atomic number on the bottom left, mass number on the top left, and charge on the top right.
What is hyphen notation?
Hyphen notation uses the element name followed by the mass number, such as sodium-23.
What is the average atomic mass?
The estimated average of the mass numbers of all isotopes of a particular element.
How do you calculate average atomic mass?
Using the formula: (Mass A)(%A) + (Mass B)(%B) + (Mass C)(%C) + ...
What is the mass number of copper-64?
64, which indicates it has 29 protons and 35 neutrons.
What is the mass number of magnesium-25?
25, which indicates it has 12 protons and 13 neutrons.
What is the shorthand notation for copper-64 with a +1 charge?
1
64
Cu+.
What is the mass of one individual atom?
The mass of one individual atom is the mass number, which is a whole number.
What does abundance refer to in chemistry?
The percentage of atoms of an element that are a specific isotope.
What is the mass number of calcium with 20 protons and 20 neutrons?
40, because mass number is the sum of protons and neutrons.
How do you calculate the average atomic mass of an element with multiple isotopes?
Multiply the atomic mass of each isotope by its percent abundance (as a decimal), then sum these values.
What is molar mass and how is it determined?
Molar mass is the mass of one mole of a substance, calculated by summing the atomic masses of all atoms in a compound.
What is the unit for molar mass?
The unit for molar mass is grams per mole (g/mol).
What is the formula for acetate?
C2H3O2-1.
What is the charge of perchlorate?
ClO4-1.
What is the formula for hydroxide?
OH-1.
What is the charge of sulfate?
SO4-2.
What is the formula for phosphate?
PO4-3.
Which transition metal has oxidation states of +2, +3, and +6?
Chromium (Cr).
What are the six strong acids that must be memorized?
HCl, HBr, HI, HNO3, H2SO4, HClO4.
What is the formula for hydrogen peroxide?
H2O2.
What is the general formula for alkanes?
CnH(2n+2).
What is the formula for methane?
CH4.
What is the formula for ethane?
C2H6.