1.1 - Monomers and polymers 1.2 - Carbohydrates
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Define monomer. Give some examples.
smaller units that join together to form larger
molecules
● monosaccharides (glucose, fructose,
galactose)
● amino acids
● nucleotides
Define polymer. Give some examples.
molecules formed when many
monomers join together
● polysaccharides
● proteins
● DNA / RNA
What happens in a condensation
reaction?
A chemical bond forms between 2
molecules & a molecule of water is
produced
What happens in a hydrolysis reaction?
A water molecule is used to break a
chemical bond between 2 molecules.
Name the 3 hexose monosaccharides.
● glucose
● fructose
● galactose
all have the molecular formula C 6 H 12 O
Name the type of bond formed when
monosaccharides react.
(1,4 or 1,6) glycosidic bond
2 monomers = 1 chemical bond = disaccharide
multiple monomers = many chemical bonds =
polysaccharide
Name 3 disaccharides. Describe how
they form.
condensation reaction forms glycosidic bond
between 2 monosaccharides
● maltose: glucose + glucose
● sucrose: glucose + fructose
● lactose: glucose + galactose
all have molecular formula C 12
H 22
O 11
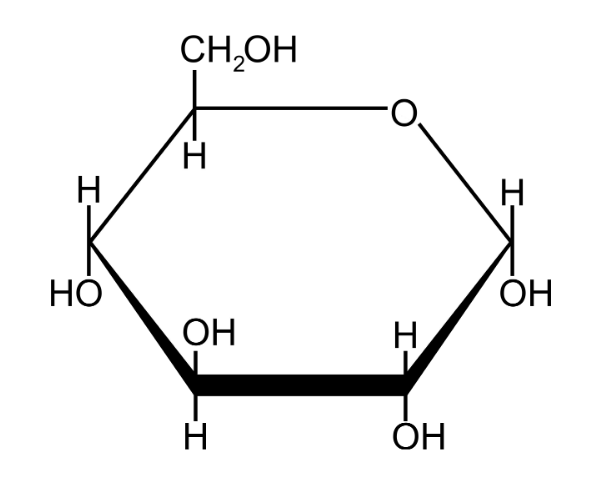
Draw the structure of ⍺-glucose.
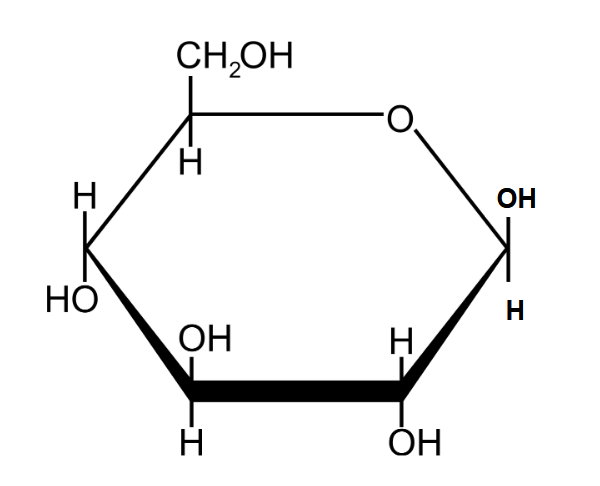
Draw the structure of 𝛽-glucose.
Describe the structure and functions of
starch.
storage polymer of 𝛼-glucose in plant cells
● insoluble = no osmotic effect on cells
● large = does not diffuse out of cells
and amylopectin:
● 1,4 & 1,6 glycosidic bonds
● branched = many terminal
ends for hydrolysis into
glucose
made from amylose:
● 1,4 glycosidic bonds
● helix with intermolecular
H-bonds = compact
Describe the structure and functions of
glycogen.
main storage polymer of 𝛼-glucose in animal cells
( but also found in plant cells)
● 1,4 & 1,6 glycosidic bonds
● branched = many terminal ends for hydrolysis
● insoluble = no osmotic effect & does not diffuse
out of cells
● compact
Describe the structure and functions of
cellulose.
polymer of 𝛽-glucose gives rigidity to plant cell walls
(prevents bursting under turgor pressure, holds stem up)
● 1,4 glycosidic bonds
● straight-chain, unbranched molecule
● alternate glucose molecules are rotated 180°
● H-bond crosslinks between parallel strands form
microfibrils = high tensile strength
Describe the Benedict’s test for reducing
sugars.
1. Add an equal volume of Benedict’s reagent
to a sample.
2. Heat the mixture in an electric water bath at
100℃ for 5 mins.
3. Positive result: colour change from blue to
orange & brick-red precipitate forms.
Describe the Benedict’s test for
non-reducing sugars.
1. Negative result: Benedict’s reagent remains blue
2. Hydrolyse non-reducing sugars e.g. sucrose into their
monomers by adding 1cm 3 of HCl. Heat in a boiling
water bath for 5 mins.
3. Neutralise the mixture using sodium carbonate solution.
4. Proceed with the Benedict’s test as usual.
Describe the test for starch.
1. Add iodine solution.
2. Positive result: colour change from
orange to blue-black.
Outline how colorimetry could be used to
give qualitative results for the presence
of sugars and starch.
1. Make standard solutions with known concentrations.
Record absorbance or % transmission values.
2. Plot calibration curve: absorbance or % transmission
(y-axis), concentration (x-axis).
3. Record absorbance or % transmission values of unknown
samples. Use calibration curve to read off concentration.