Dr ME - Stoichiometry, acids and bases
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
40 Terms
Define stoichiometry
Calculations relating the amount of products and reactants in chemical reactions
Define theoretical yield
Maximum mass of product that can be obtained assuming all of the limiting reagent is consumed.
Define actual yield
Amount of product actually obtained in the experiment. Can only be determined by doing the experiment.
Equation to calculate percentage yield

Define percentage purity
In a given mass of material it is the percentage amount (mass) of the material of interest
What does the Valence shell electron pair repulsion theory suggest?
Predicts the shape based on tetrahedral - 2 bonding pairs and 2 lone pairs of electrons
Lone pairs are more sterically demanding (bigger) that bonding pairs, so the bonding pairs are pushed closer together.
What is the Arrhenius theory of acids and bases
Acids release H+ ions into water
Bases release OH- ions into water
Bronsted-Lowry definitions of acids and bases
Acids - H+ donors
Bases - H+ acceptors
Most acids and bases fit the Arrhenius equation except ammonia - it has basic properties but it isn’t the source of OH- ions.
Define amphoteric
Can act as either acid or base
Define amphioprotic
Can accept or donate H+
Define Lewis acids and bases
Acid - electron pair acceptor
Base - electron pair donor
Define ligand
Species that can donate an electron pair to the metal i.e. any lewis base
Define coordinate bond
A bond in which both the shared electrons come from one of the atoms.
Explain the 2 factors that affect acid strength
H-A Bond strength - If the bond is strong then the acid will be weak as energy is required to break the H-A bond.
If proton is bonded to a more electronegative atom the acid will be stronger.
Because in H - A if A is more electronegative there is a stronger pull on the shared electron.
There is a more polar bond
Easier to break the bond
What are binary acids?
Acids formed by halogens e.g. HF, HCl, HBr, HI
HI Has the weakest bond so dissociates more easily and is the strongest acid.
Bond strengths decrease from F to I and acid strength increases from F to I.
What are hydrides of 1st row elements?
CH4, NH3, H20, HF
Large increase in electronegativity from C to F leads to increasing polarity of the bond from C to F so easier bond dissociation.
Carboxylic acids
They have neighbouring polar C=O that increases the polarity of O-H. They are medium weak acids.
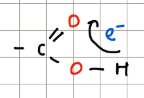
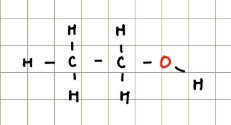
Alcohols
Contain a polar OH group but have no neighbouring C=O groups so they’re very weak acids.
Structure and name of HNO2
Nitrous

Structure and name of HNO3
Nitric
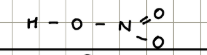
Name and structure of H2SO3
Sulforous
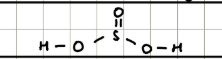
Name and structure of H2SO4
Sulphuric
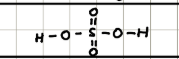
Name and structure of H2CO3
Carbonic
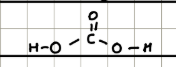
Name and structure of HOCl
Hypochlorus (bleach)
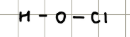
Name and structure of HClO2
Chlorous
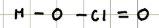
Name and structure of HClO3
Chloric
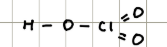
Name and structure of HClO4
Perchloric
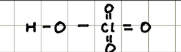
Name and structure of H3PO4
Phosphoric
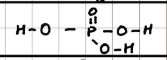
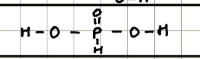
Name and structure of H3PO3
Phosphorus
Three factors affecting acidity
Oxidation number of central atom
Normally positive
If large and positive, it exerts a strong pull on electrons shared with oxygen.
Size of central atom
For central atoms with the same ox number, the e withdrawing effect is greater for the smaller ion as it has a higher charge density.
Electronegativity of central atom
A measure of how strongly it attracts the e.
What is an indicator?
It is a large water soluble weak acid or base that changes colour in some known pH range.
Methyl Orange
Red to yellow
3.2 - 4.2
Methyl Red
Pink to yellow
4.2 - 6.2
Bromothymol blue
Yellow to blue
6.0 - 7.6
Phenolphthalein
Colourless to red
8.0 - 10.0
Equivalence point
The point of chemical equivalence when the number of mols of acid = number of mols of base
End point
Point at which the titration has stopped
What is a primary standard?
A very pure compound that can be accurately weighed out to give a reliably known number of moles from which a solution can be made up. The concentration of this solution is then reliably known, so can be used to standardise other solutions.
Requirements of primary standards
High purity
Air stable
No hydrate water
Cheap and readily available
Adequate solubility
Reasonably large formula weight - reduces relative error on weighing
What is the solvent levelling effect?
Where a solvent limits the apparent strength of very strong acids or bases dissolved in it, making them appear equally strong by reacting completely with the solvent.