Week 5 (apoptosis + Cancer)
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
What is apoptosis?
programmed cell death
Controlled cellular suicide
Dehydration leading to cell shrinkage (allows for other cells to digest the remains)
Not the only way for cell death:
Autophagy, non-apoptotic programmed cell death/caspase-independent, Anoikis, cornification, excitotoxicity, & necrosis
What are the changes related to apoptosis
Include morphological & chemical changes
Morphological = membrane blebbing/shrinkage & condensation (cells break apart with intact membranes to form apoptotic bodies), cytoskeletal collapse, disassembly of nuclear envelope (condensed nucleus), & chromatin fragmentation
Chemical = cell surface is altered for phagocytic recognition & intracellular proteins act as signals and activators of cell destroyers
What causes apoptosis
Causes: stressed out cells die when there is...
Radiation
Glucocorticoid binding of nuclear receptors (cortisol = stressful environment)
Heat
Nutrient deprivation
Viral infection
Hypoxia (lacking enough oxygen)
High intracellular calcium
What is necrosis
cell death in response to acute issue
Explosion like
Not controlled
What is caspase
protease with a cysteine at their active site that cleave target substrates at a specific aspartic acid
Plays a role in both intrinsic and extrinsic pathway
What is the intrinsic signaling pathway (simple)
Intrinsic signaling pathway = internal response to injury or hypoxia
Depend on cytochrome c release
Requires Bcl2
Sometimes recruited by the extrinsic pathway
What is the extrinsic signaling pathway (simple)
Extrinsic signaling pathway = extracellular signal protein binds to death receptor on cell surface
Sometimes recruits intrinsic pathway
What is a death receptor
Death receptor = activate caspase apoptosis (extrinsic pathway)
Have a ligand-binding region, 1 transmembrane domain, & a death domain
Belong to the TNF receptor family
Binding ligands belong to the TNF signal protein family
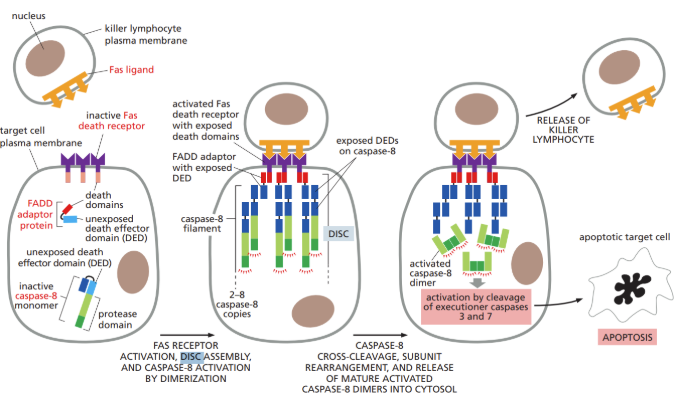
What is DISC
DISC = death-inducing signaling complex
Complex in which initiator caspases interact and are activated after extracellular ligands bind to cell-surface death receptors in the extrinsic pathway of apoptosis
What is cytochrome c
water soluble molecule that can trigger apoptosis
Released by the mitochondria
Trigger intrinsic pathway
Can induce apoptosis, independent of its electron-transport activity
What is BH123 protein
BH123 protein = pro-apoptotic protein (Bak & Bax) (BH3-only similar)
Regulator of cell death
What is Bcl2 protein
Bcl2 protein = anti-apoptotic protein located on organelle membranes that acts as a membrane guardian
Regulator of cell death
Block cell death
What are survival factors
Survival factor = extracellular signals from other cells to avoid apoptosis
Cells with no signals cannot import nutrients. Therefore, they eat themselves (autophagy) but eventually starve to death (not apoptosis)
Can stay alive by
Increasing anti-apoptotic proteins (Bcl2)
Inactivating pro-apoptotic proteins (BH3-only and BH123)
What is autophagy
Autophagy = chewing up the cell insides (can be a form of cell death)
Cell eats itself
Why is programmed cell death needed
Why programmed cell death is needed...
Quality control during development for abnormal cells
Development = more cells than needed are made just in case they have to be eliminated
Organ/tissue sculpting
Maintenance of short-lived cell supply
Organelle damage
Elimination of bad immune system cells, unneeded activated lymphocytes
What is an example of normal apoptosis
elimination of cells between developing fingers and toes in a human embryo
What is the importance of phosphatidylserine flipping out in an apoptotic cell
Chemical changes with apoptosis:
DNA fragmented by endonuclease at the linker regions between nucleosomes
Membrane phospholipid phosphatidylserine changes conformation and moves to outer leaflet of lipid bilayer
Release of mitochondrial cytochrome c into cell cytosol
Vacuole formation related to organelle autophagy
What is caspase and its role
Caspases = proteases with a cysteine at their active site and that cleave target substrates at a specific aspartic acid
Located in cytoplasm as inactive procaspases
Activated procaspases become either initiator caspases or executioner caspases
They cleave specific proteins (like lamins, endonucleases, cell-cell proteins, & cytoskeletal proteins) to either start or stop their function
What is caspases role in activation (steps)
Activation:
2 signal pathways (extrinsic or intrinsic)
Steps:
Receive a death signal
Procaspase domains with recruitment domain allows them to assemble with adaptor proteins into activation complexes
Proximity of initiator procaspases activates them (they cleave each other) BEGINS THE CASCADE
Executioner procaspases are activated and death signal is amplified
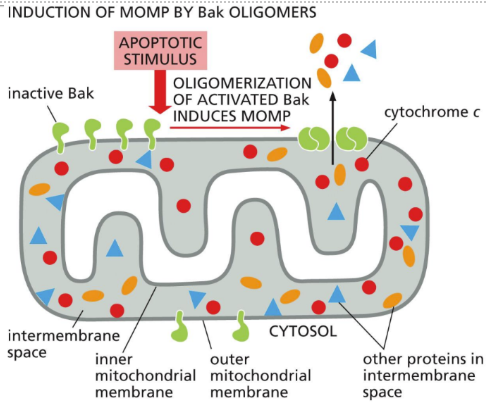
What is the intrinsic pathway (detailed)
Intrinsic = internal response to injury or hypoxia triggers apoptosis
Dependent upon release of cytochrome c and other proteins from mitochondria which activate a caspase cascade
Proteins bind to adaptor protein Apaf1 which becomes an apoptosome which activates procaspases
Sometimes recruited by the extrinsic pathway
Requires Bcl2 (anti-apoptotic protein)
BH3-only first, then Bcl2 anti-apoptotics, then effector proteins
Can be activated by p53 (tumor suppressor)
If cellular DNA damage accumulates
Will activate other proteins & trigger the intrinsic pathway
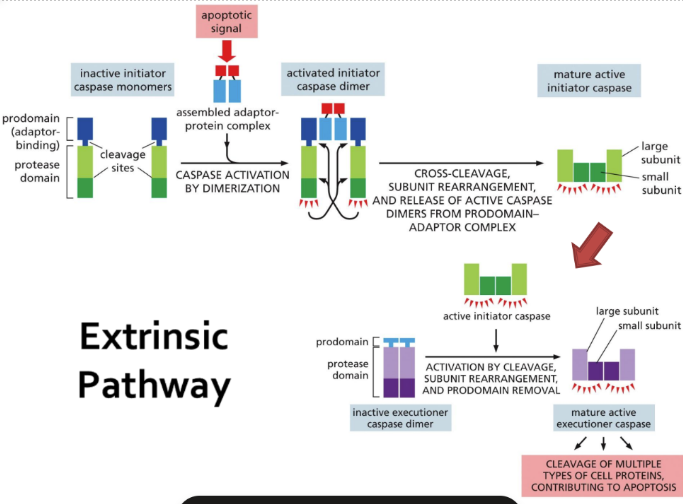
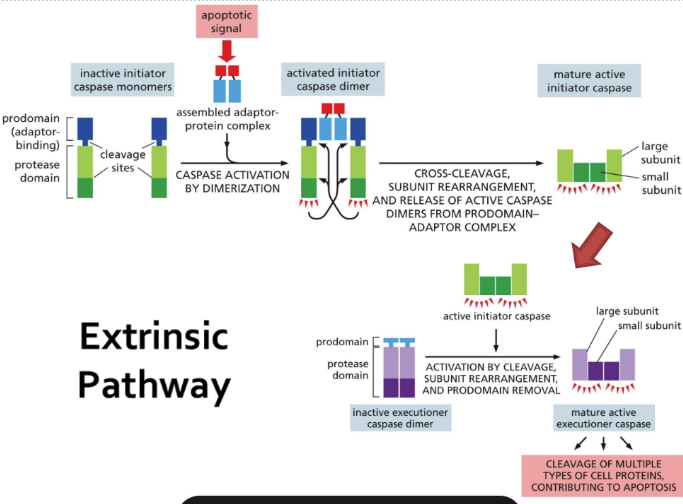
What is the extrinsic pathway (steps detailed)
Extrinsic = extracellular signal proteins bind to death receptors on a cell surface
Binding initiates cascade of events within the cell
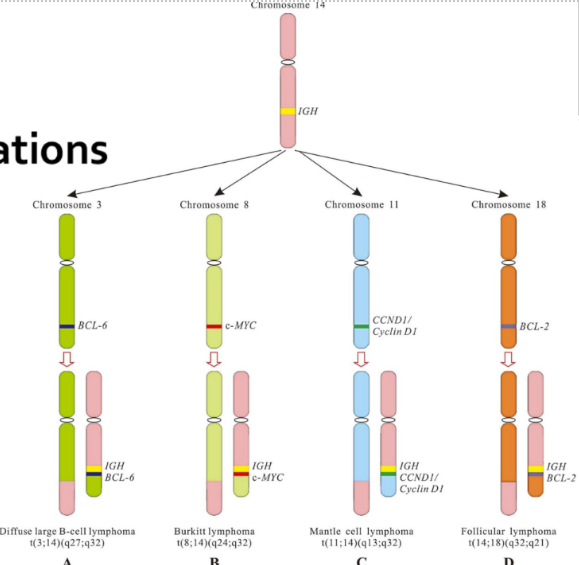
What is cancer’s role with apoptosis
Cancer = tumorigenic cells regulate the apoptotic pathway abnormally
Bcl2 production increases after chromosome translocation -> leading to apoptotic inhibition of the cells (lymphocytes)
P53 gene mutated so there’s no cell death or cell-cycle arrest => tumors (very common in many cancers)
Cells lose adherence to neighbor cells
image shows translocation examples
What is angiogenesis
Angiogenesis = Growth of new blood vessels by sprouting from existing ones
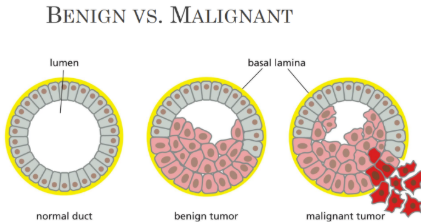
What is benign mean
Benign = mass of cells/tumor that is not invading
What is cancer stem cells
Cancer cells lose their differentiation, therefore, they look like stem cells
What is a carcinoma
Carcinoma = cancers arising from epithelial cells
Most of our cells are these so 80% of cancers are these
Adenoma = benign tumor arising from epithelial cells in a gland
What is a carcinogen
a substance or agent that can cause cancer (chemical, viruses, UV damage)
What is cancer
a collection of disorders that share 2 properties: cell reproduction & division despite various restrains and controls & invasion
metastasis
What is neoplasm
Neoplasm = mass of cells or tumor
No invasion when benign
What is a malignant neoplasm
Malignant neoplasm = made up of cells from a single ancestor
Monoclonal in origin and form the “primary tumor”
Invade nearby tissues
What is leukemia (Blood)/lymphoma (immune system)
WBCs and immature precursors proliferate (blood cancer)
What is a sarcoma
Sarcoma = cancers arising from connective tissue or muscle cells
What is metastasis
Metastasis = when cells are malignant and invade (THIS IS CANCER)
Spread to more distant sites in the body to form secondary tumors
Fewer than 1 in 1000 cells will survive and metastasis successfully
What is a tumor suppressor
Gene that appears to help prevent formation of a cancer. Loss-of-function mutations in such genes favor the development of cancer
What is an oncogene
An altered gene whose product can act in a dominant fashion to help make a cell cancerous. Typically, an oncogene is a mutant form of a normal gene (proto-oncogene) involved in the control of cell growth or division
Originate from proto-oncogenes that encode protein products that control cell growth and differentiation
When activated by mutation, these act as dominant gain-of-function mutations that lead to the deregulation of cell cycle control
A growth promoting effect
Ex: Myc, K-ras, Wnt-3, her-2/neu, cyclin E
Still a normal protein, it is just being over produced
ex: p53, CDK inhibitors, BRCA1
What is a proto-oncogene
Proto-oncogene = Normal gene, usually concerned with the regulation of cell proliferation, that can be converted into a cancer-promoting oncogene by mutation
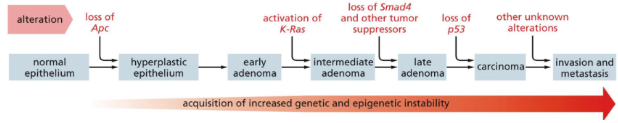
What is the basic cause of cancer
Basic cause of cancer = Damage to specific genes (mutations) that accumulate in somatic cells (somatic mutations) over time until a cell loses a critical number of growth-controlled mechanisms and initiates a tumor
Mutations involved in regulating cell growth & differentiation may occur and can lead to deregulation of growth and cell cycle madness
If only one mutation were able to convert healthy cells into cancerous cells, we would not be viable organisms (need multiple mutations for cancer to develop)
At least 5 mutations are required for onset of clinically observable tumors:
Gene amplification: extra copies of a single gene are transcribed
Nonsense mutations
Gene deletion: loss of a sequence of nucleotides within an exon or splice site
Gene rearrangements: in Ig-producing cells (T and B) can produce massive clonal populations
Point mutations: replacement of a single nucleotide
Other causes include genetic alterations of specific molecules such as integrins or chromosome issues
Specific genetic alterations are associated with specific caners
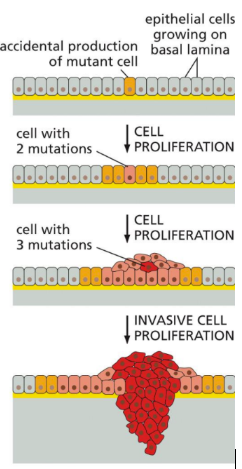
What are tumors
Tumors: comprised of genetically identical cells and are clonal in nature
Single aberrant cell starts it all
Alterations in cellular DNA accumulate over time in succeeding generations of daughter cells (clonal expression)
Daughter cells with several mutations replace the cells previously comprising the tumor
Induce angiogenesis
What are cancer cell characteristics
Cancer cells: are genetically unstable & continue to accumulate more point mutations or deletions or develop crazy chromosome issues more than normal cells
Have abnormal growth control
Lose their differentiation (look like stem cells)
Have defective death control
Utilize glucose at a much higher level (suck energy of neighboring cells)
Continue dividing despite DNA damage
Avoid replicative senescence through p53 mutation or telomerase maintenance
Must survive in a foreign environment
Critical genes = proto-oncogenes/oncogenes, tumor suppressor genes, & DNA maintenance genes
What is angiogenesis (detailed)
Angiogenesis:
Cell aggregates need oxygen to survive, without oxygen, hypoxia sets in
Hypoxia activates an angiogenic switch that increases hypoxia-inducible factor that activates transcription of genes that encode proteins that attract endothelial cells (via VEGF) and formation of new blood vessels
Tumors may function like this
What are tumor suppressor genes (detailed)
Tumor suppressor genes = Gene that appears to help prevent formation of a cancer. Loss-of-function mutations in such genes favor the development of cancer
Encode for protein products that suppress tumor formation by controlling cell growth
Loss-of-function mutation = results in inactivation of the tumor suppressor protein and can lead to uncontrolled cellular proliferation
Ex: p53, BRCA1, E3 ubiquitin protein, RB, p16, CDK inhibitors
Inactivation of these genes occurs through mutation or deletion of sequences over a large coding region (exon)
Causes uncontrolled cellular proliferation because of loss of negative regulation
Like the brakes on the bus
What is p53 and it’s role with DNA damage
p53 = a cellular stress sensor that reacts to various stresses and produces specific responses that stop damaged cells from dividing (guardian of the genome)
Tumor suppressor gene & DNA repair gene
Only functions in certain circumstances, limiting the harm done by DNA damage
Can set apoptosis in motion
Stops cell division and cell cycle
Induces transcription of p21
Loss of p53 promotes cancer
mice without p53 are normal but develop cancer before reaching 10 months old
Promotes cancer by:
Allowing DNA-damaged cells to divide
Allows damaged cells to escape apoptosis
Leads to genetic instability
Makes cells resistant to anti-cancer drugs
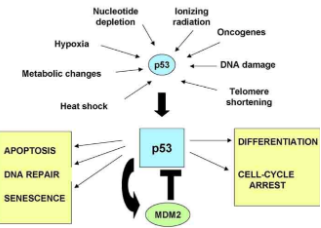
What are Cdk inhibitors
Cyclin-dependent kinase inhibitors = one type of protein coded for by tumor suppressor genes
Inactive cyclin-dependent kinases and prevent them from phosphorylating (therefore controlling cell cycle)
What are DNA repair genes
DNA repair genes = genes that assure accurate replication of DNA
Ex: p53
If mutated, these genes that encode specific proteins causes genomic instability (widespread mutations, chromosome breaks, aneuploidy)
When these mutations affect pathways that regulate cellular proliferation, a tumor may arise
What are proto-oncogenes (detailed)
Proto-oncogenes = Normal gene, usually concerned with the regulation of cell proliferation, that can be converted into a cancer-promoting oncogene by mutation
Necessary for normal cellular proliferation (originally obtained from viruses)
Become activated by point mutations to become an oncogene
Like the accelerator on a bus (destination = cell division)
What causes cancer cells to be immortal
Cancer cell immortality:
Tumor cells overcome replicative cell senescence (cell death after a set number of divisions) by activating telomerase which replaces the telomere segments that are lost during each cell division
Changes in cancer cells disable checkpoint controls so cell cycle never stops
Telomerase activity is maintained