Chem H Bonding UNIT
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
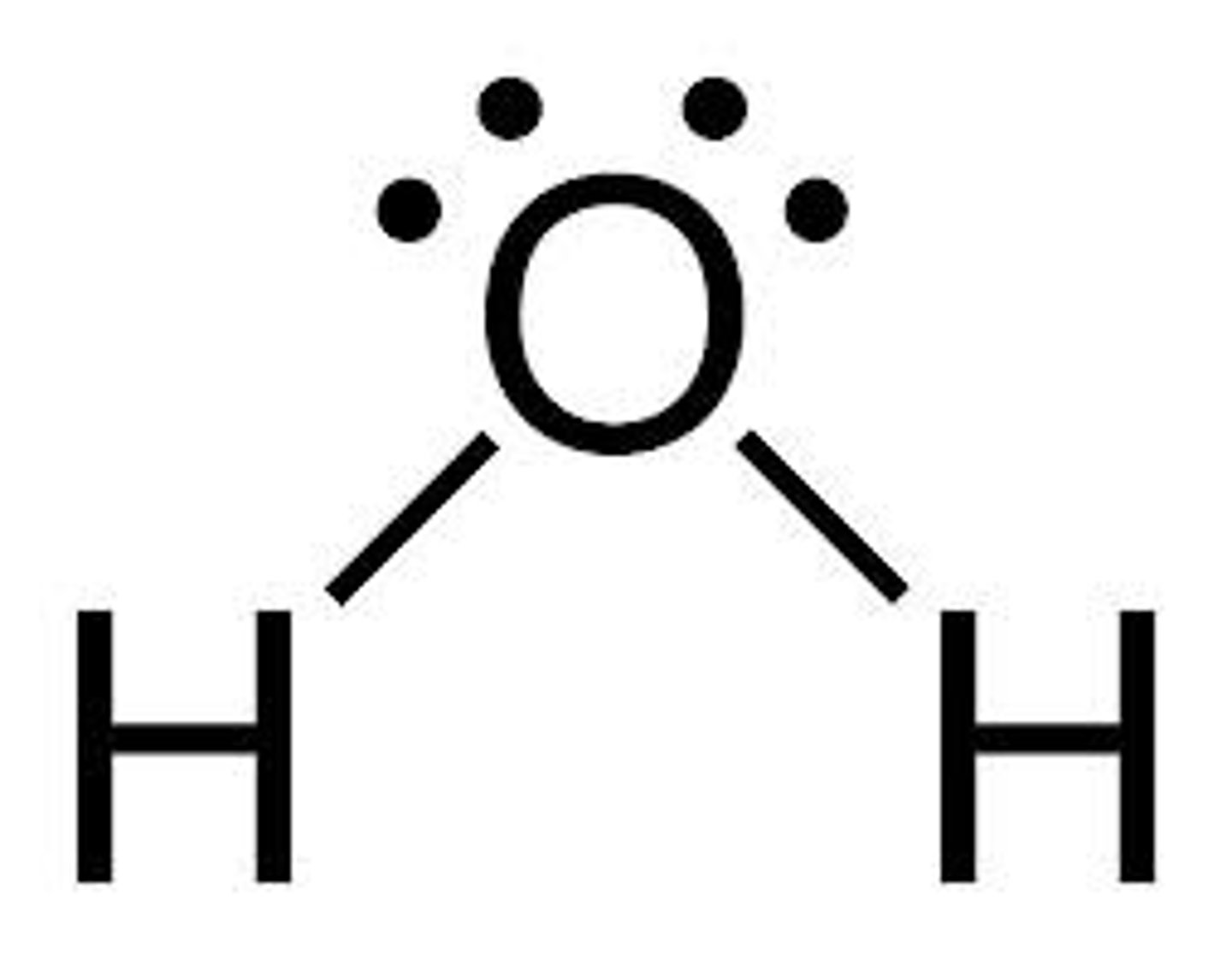
H2O lewis structure
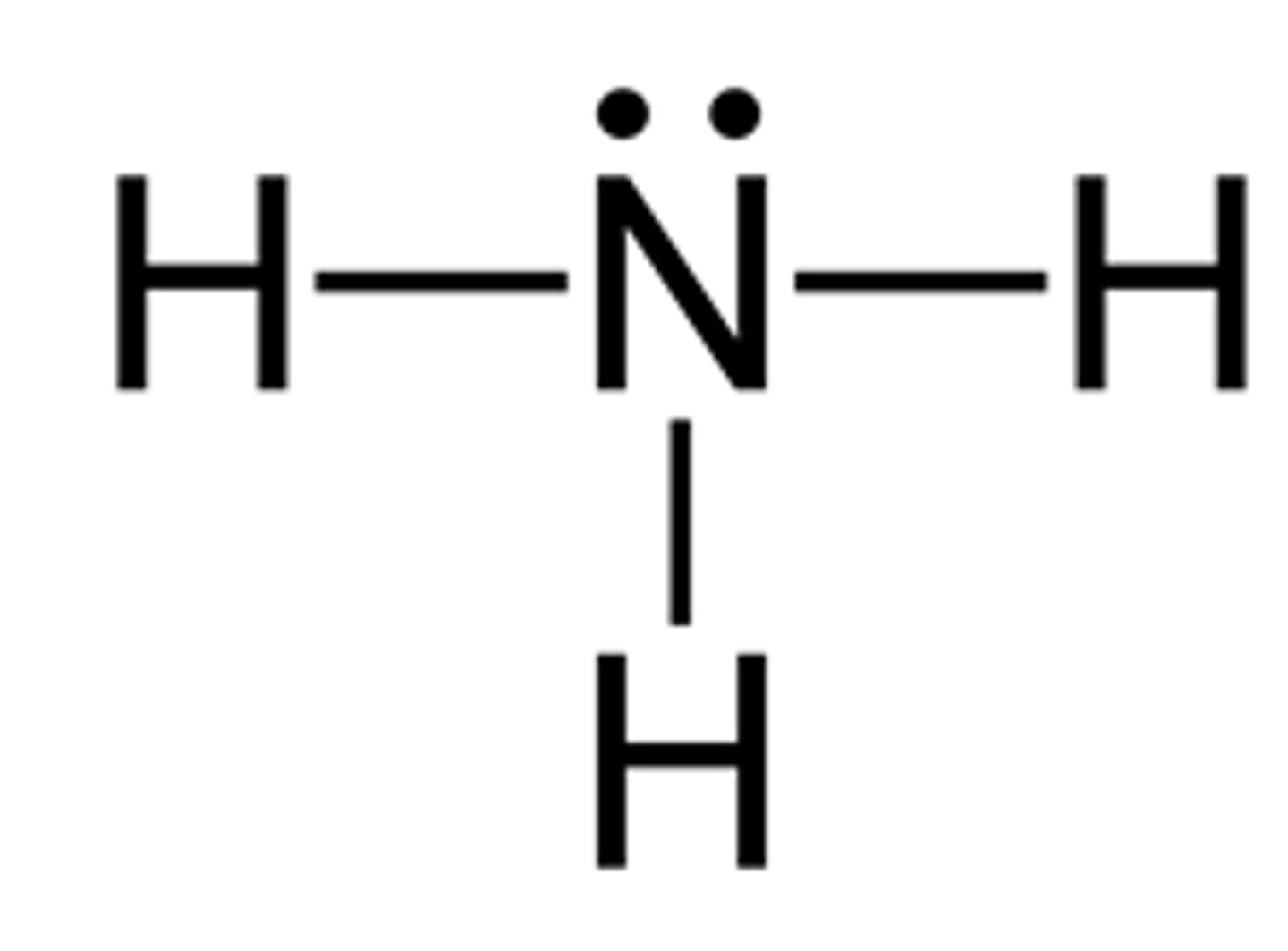
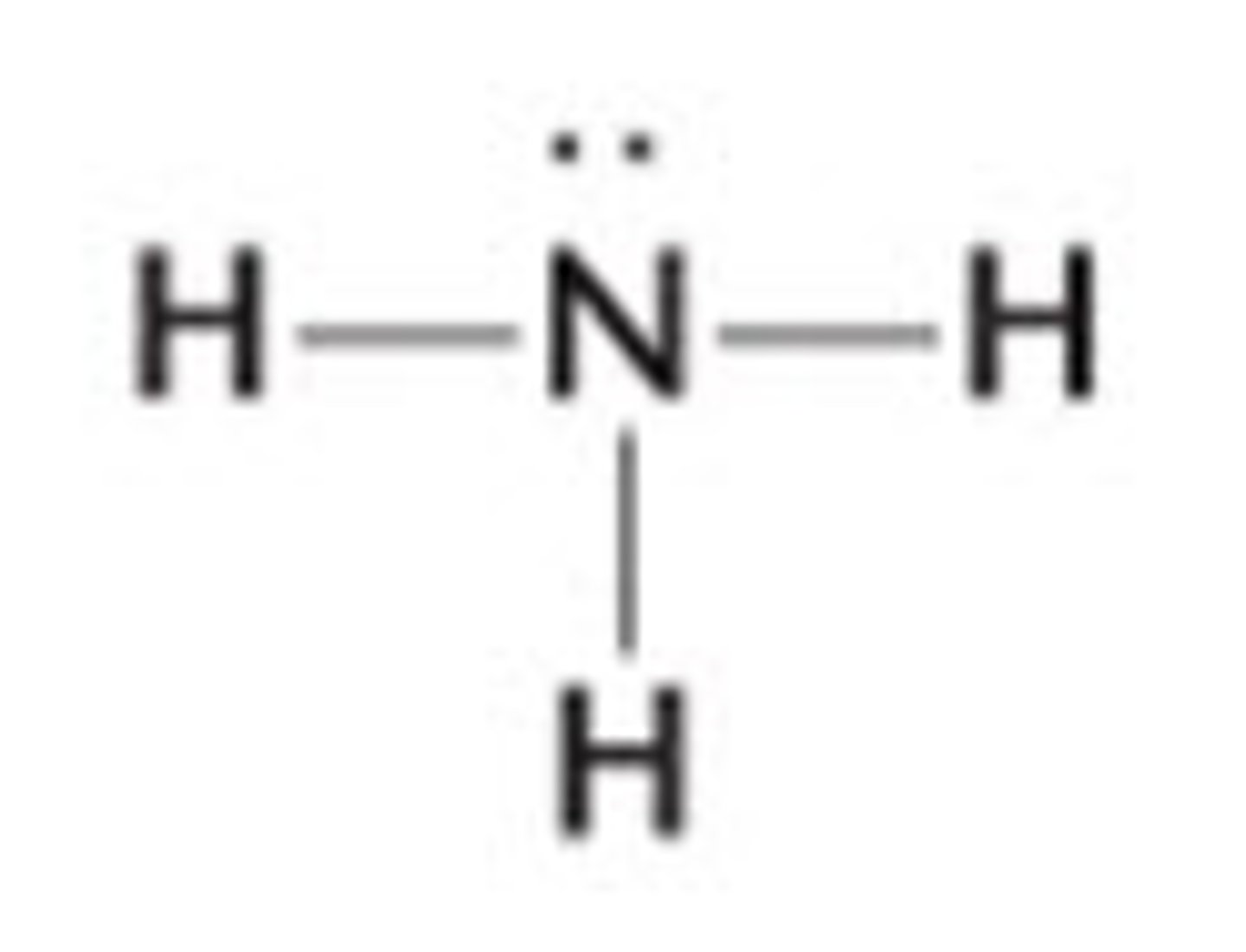
NH3 lewis structure
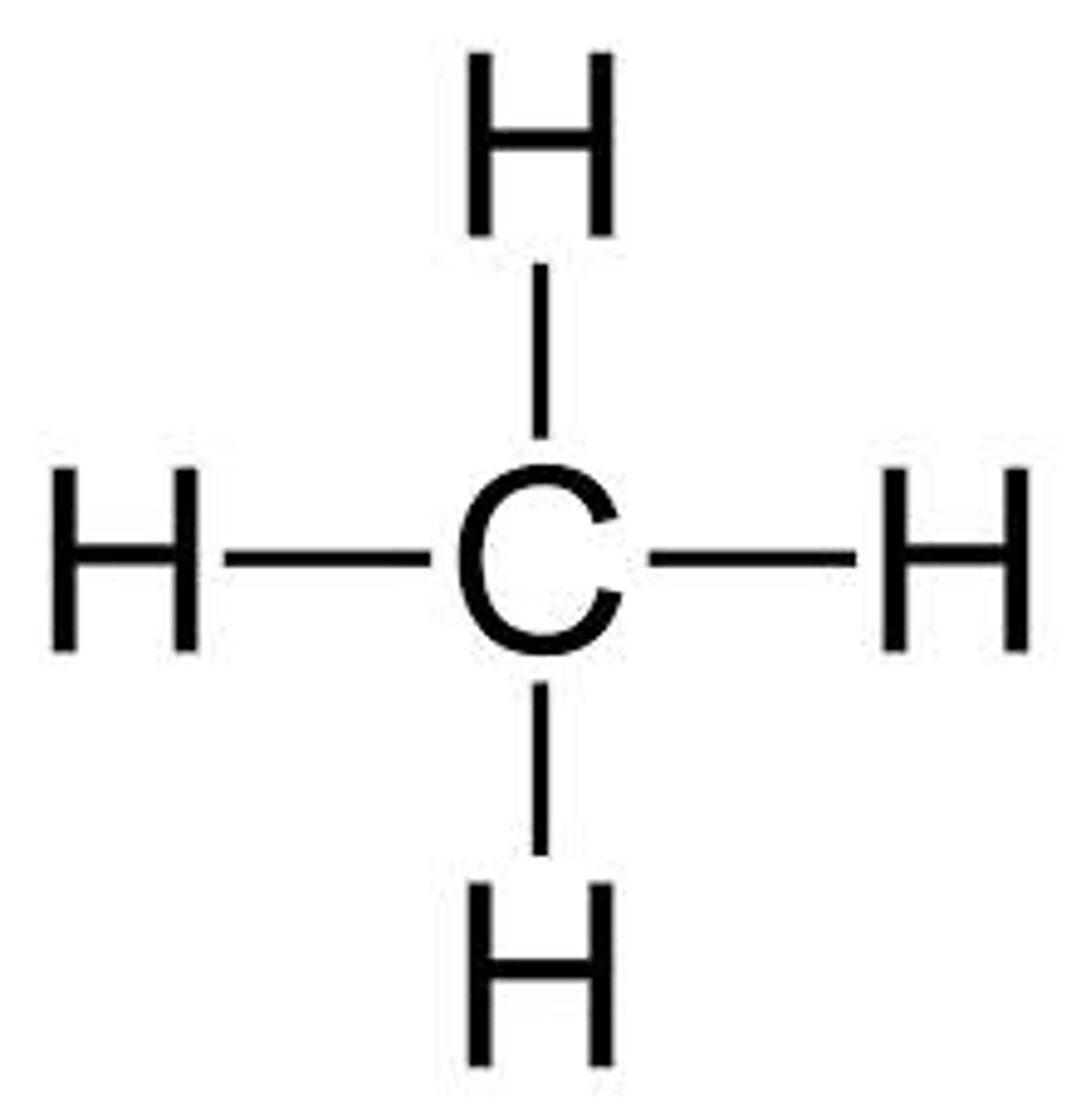
CH4 lewis structure
H2 lewis structure
H-H
N2 lewis structure

O2 lewis structure
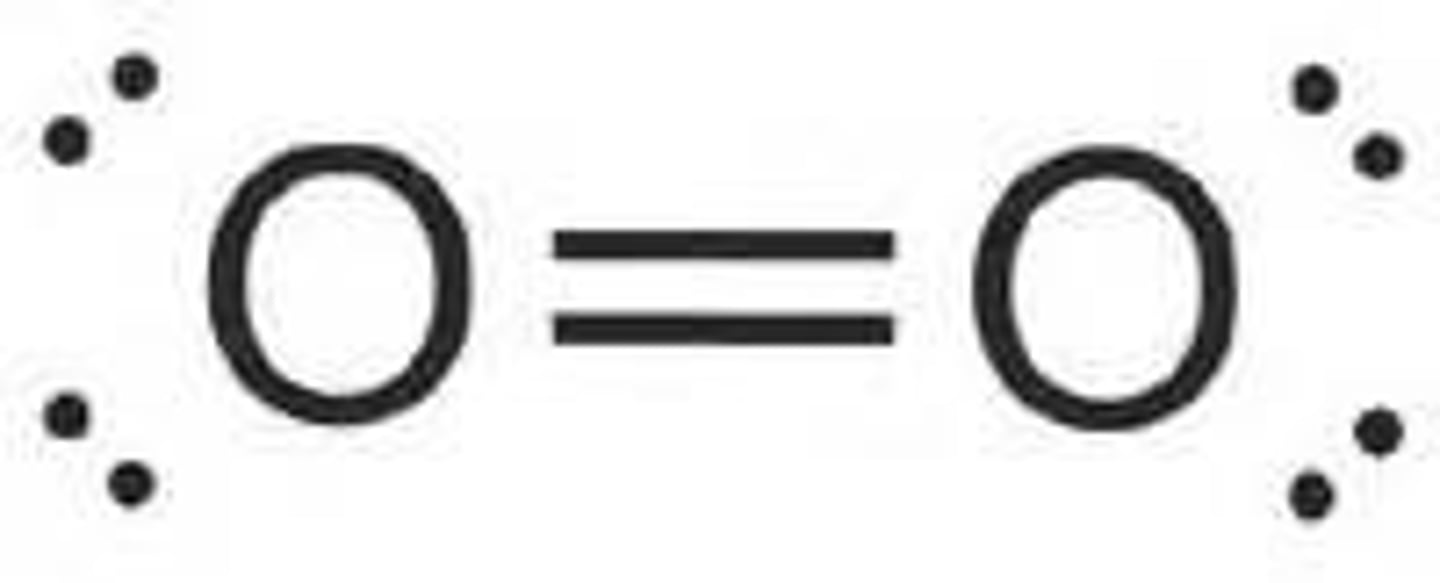
CO2 lewis structure
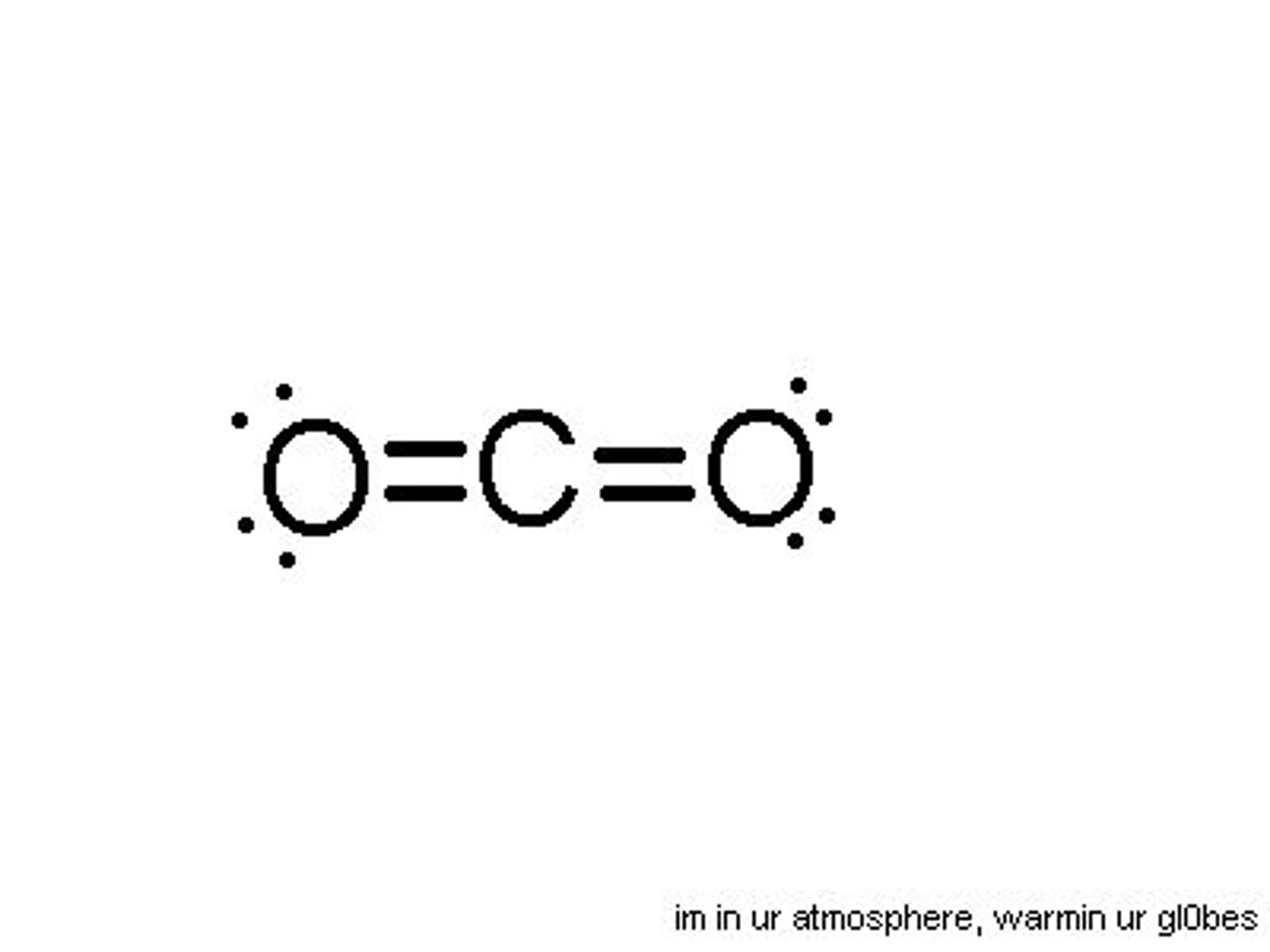
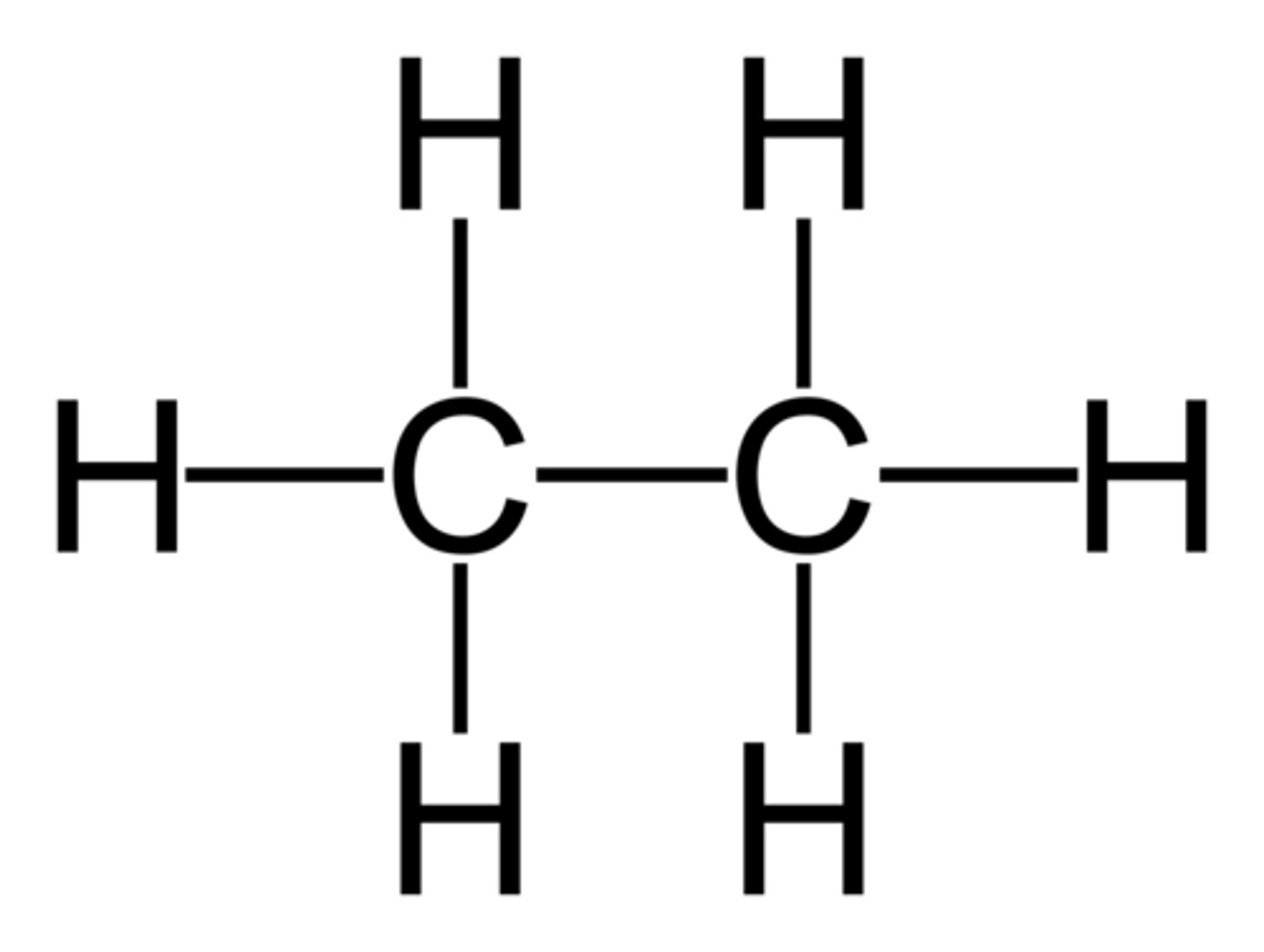
C2H6 lewis structure
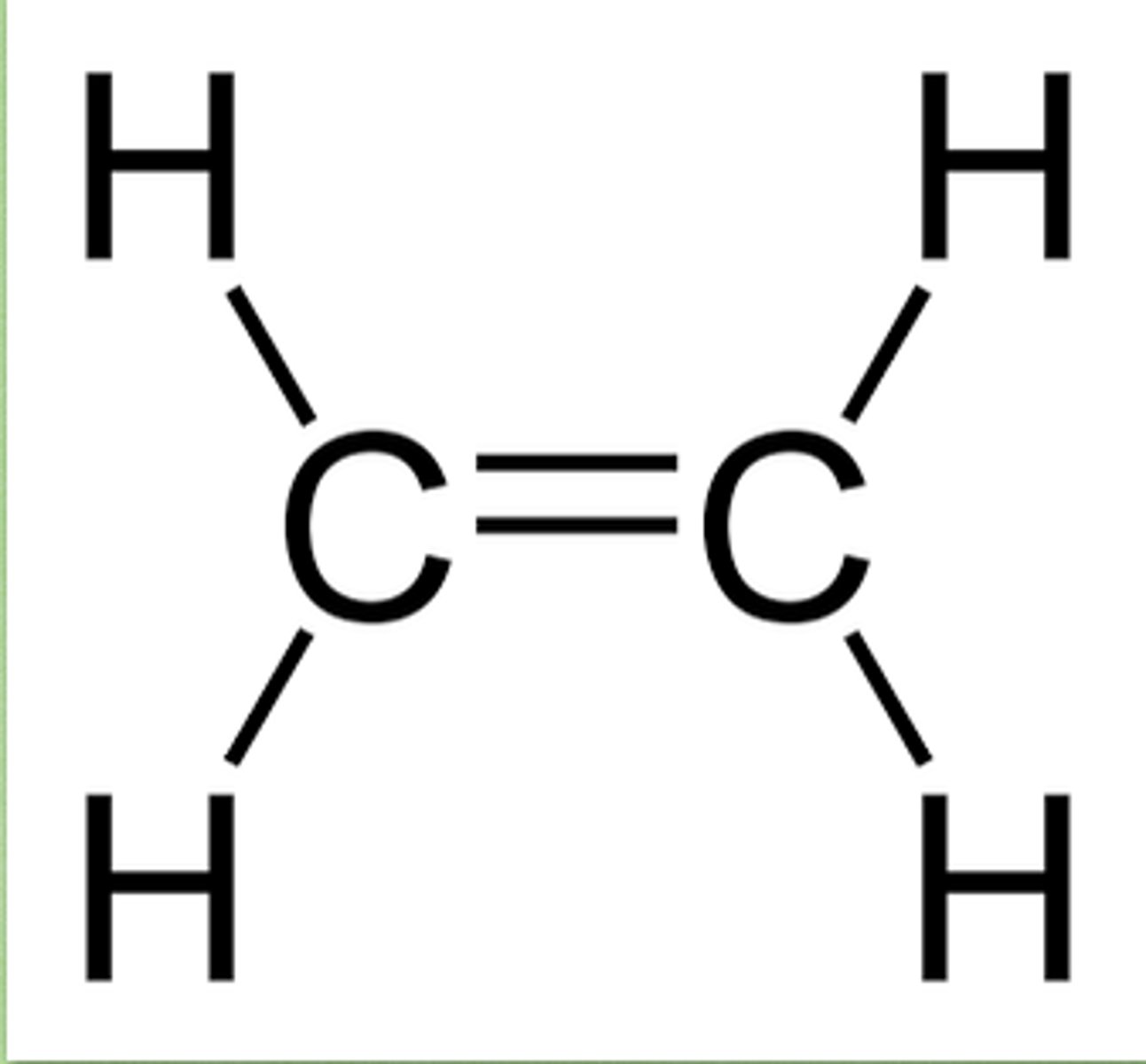
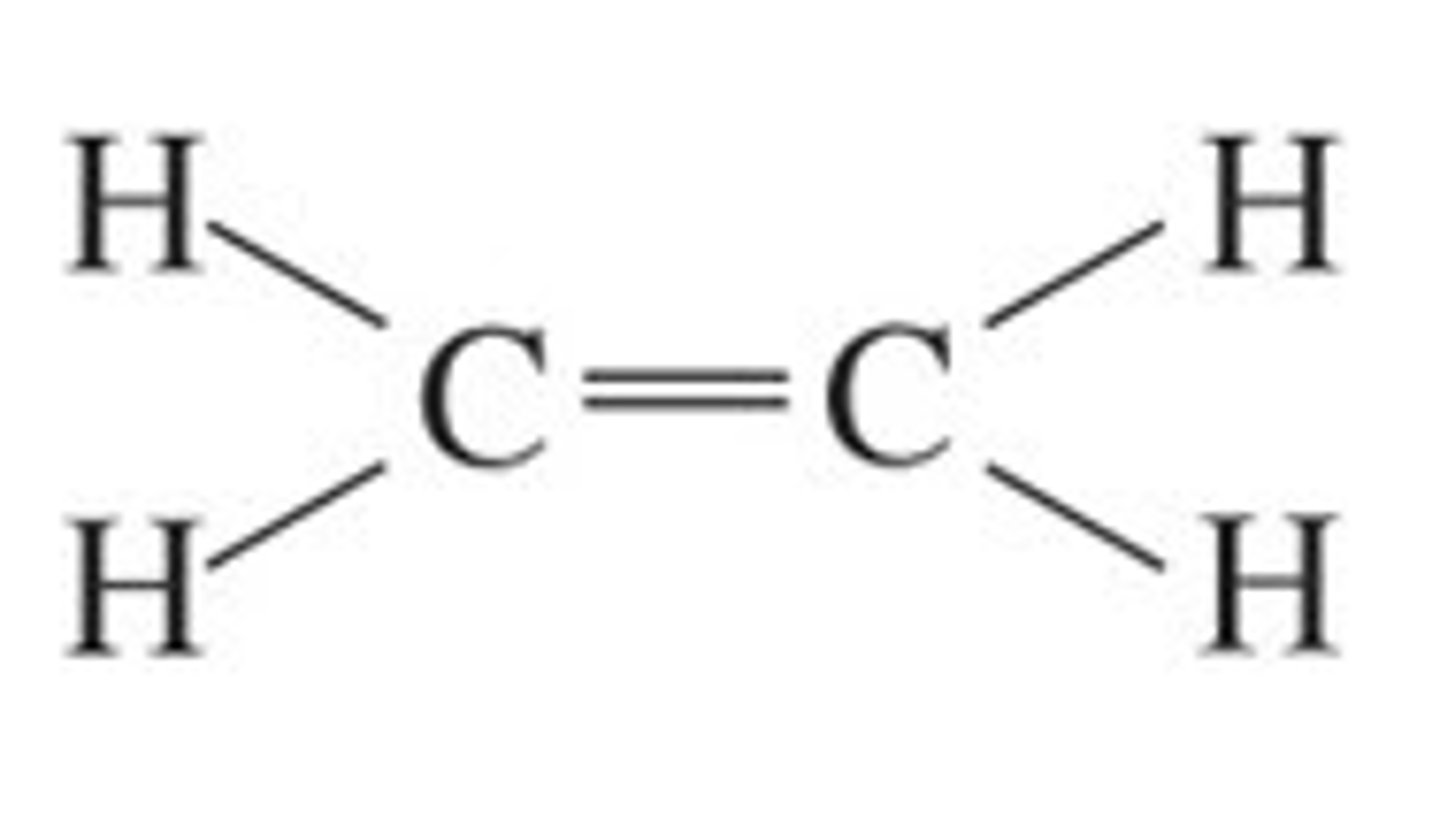
C2H4 lewis structure
C2H2 Lewis Structure
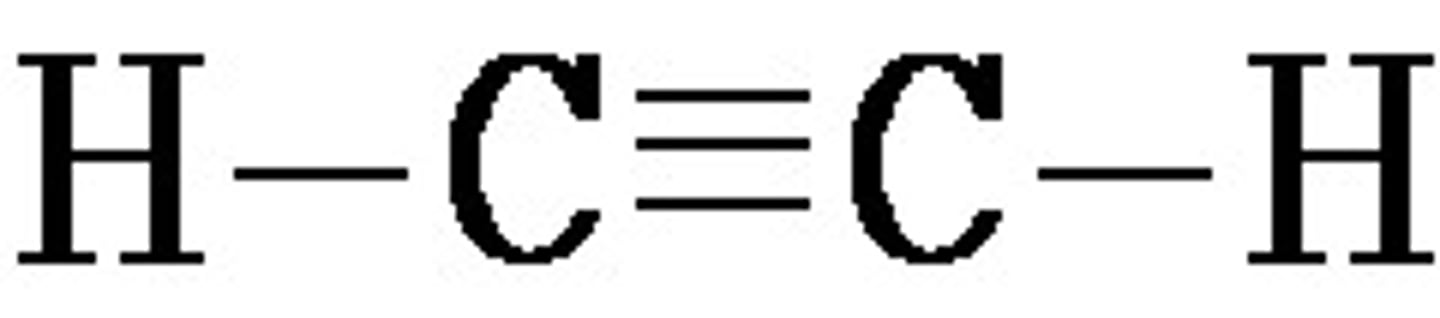
C2H5OH lewis structure
but 4 dots on O
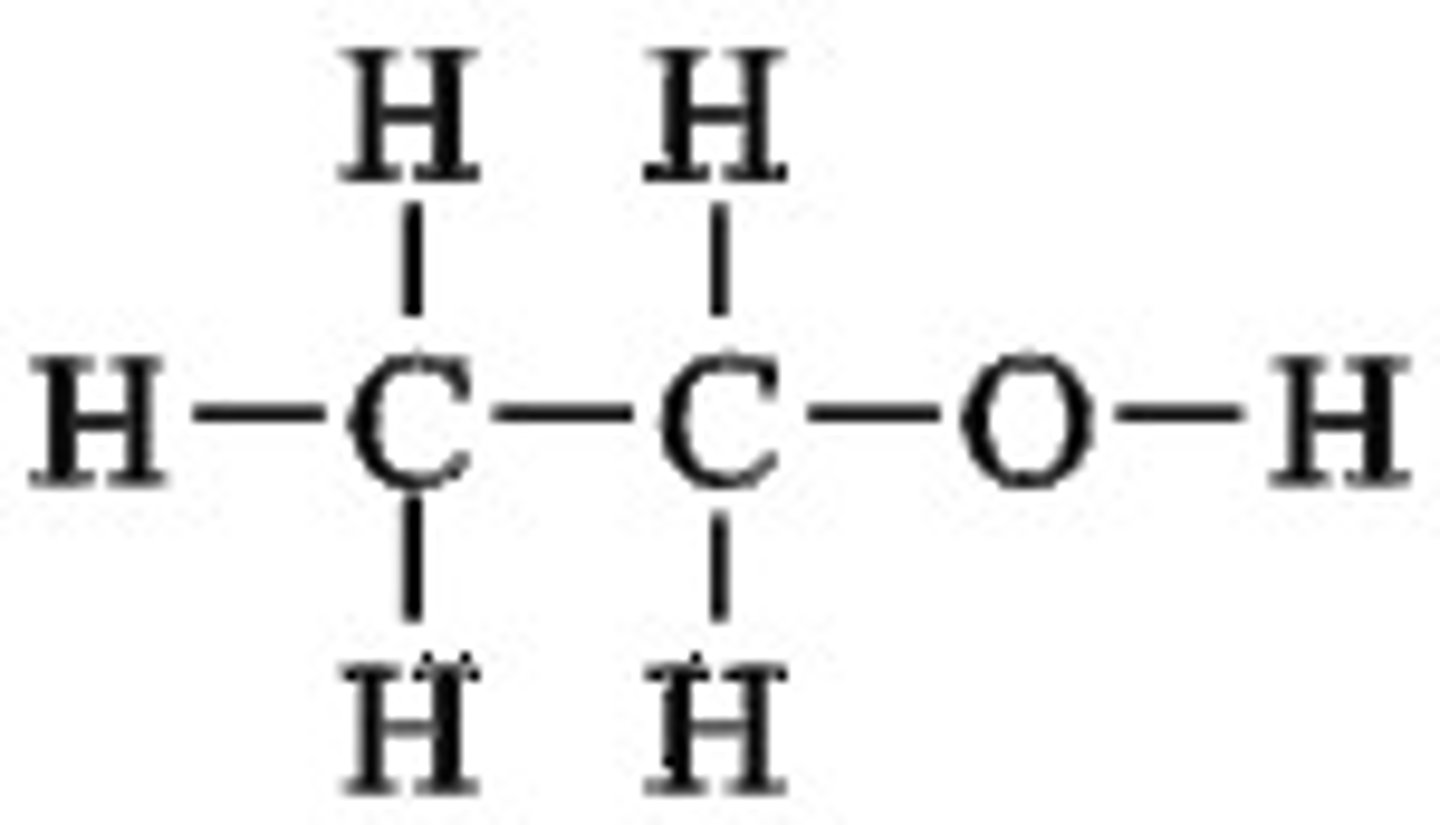
Br₂
linear, nonpolar
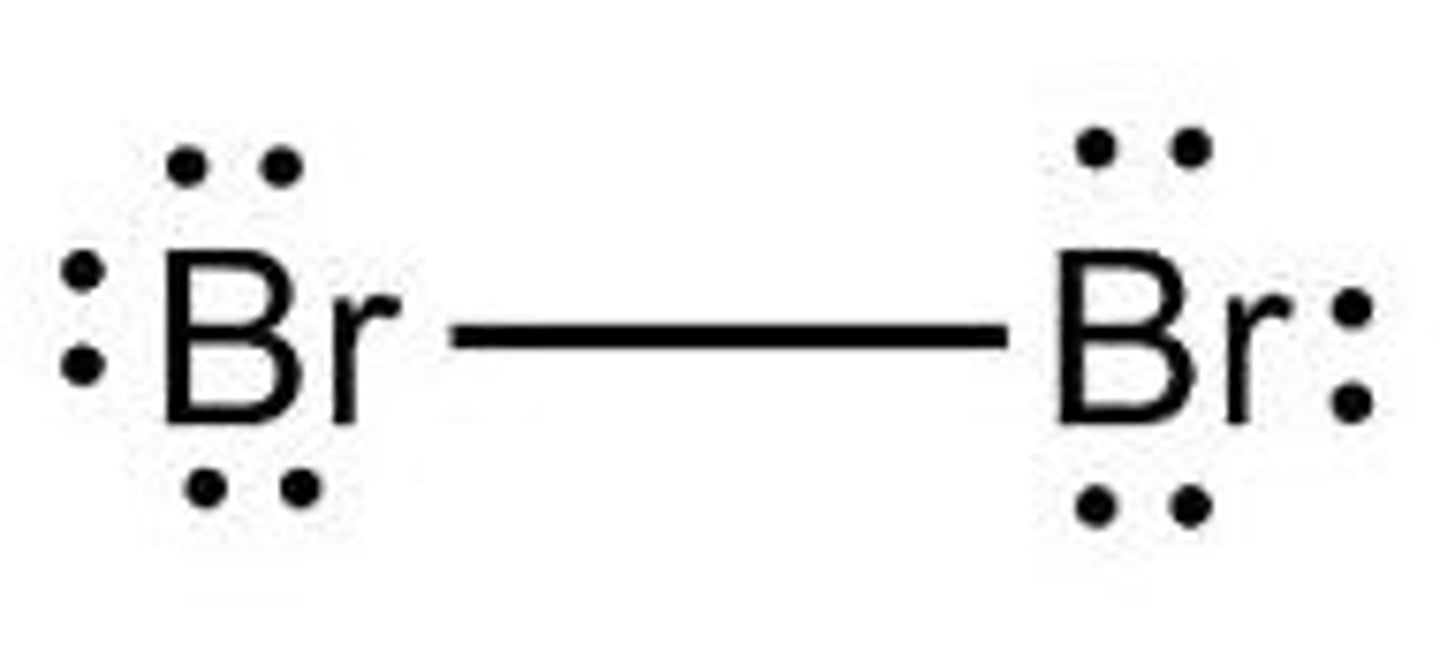
C₂H₂
linear, nonpolar
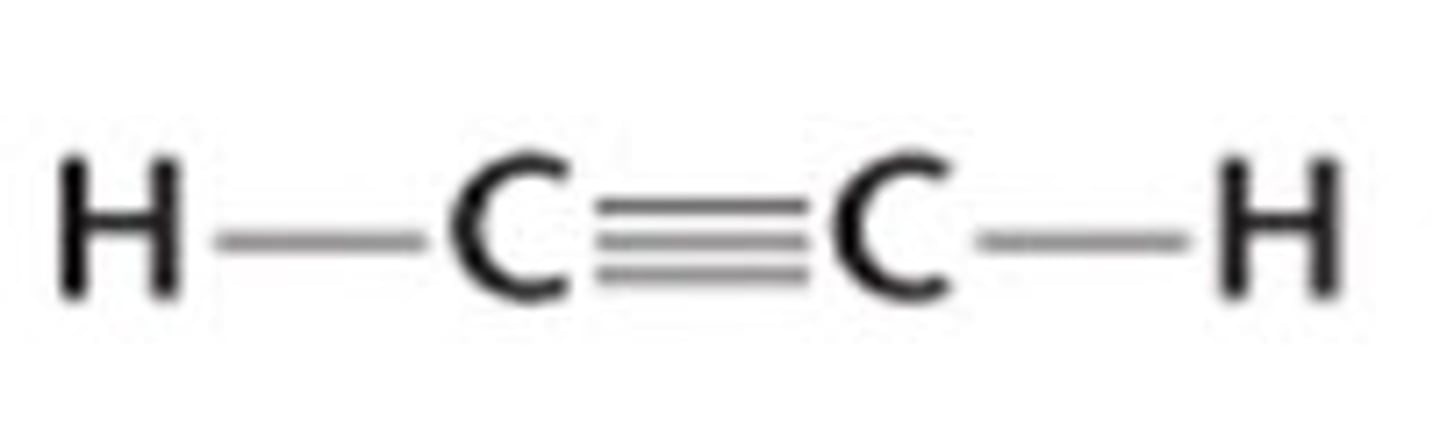
C₂H4
trigonal planar (C-H), nonpolar
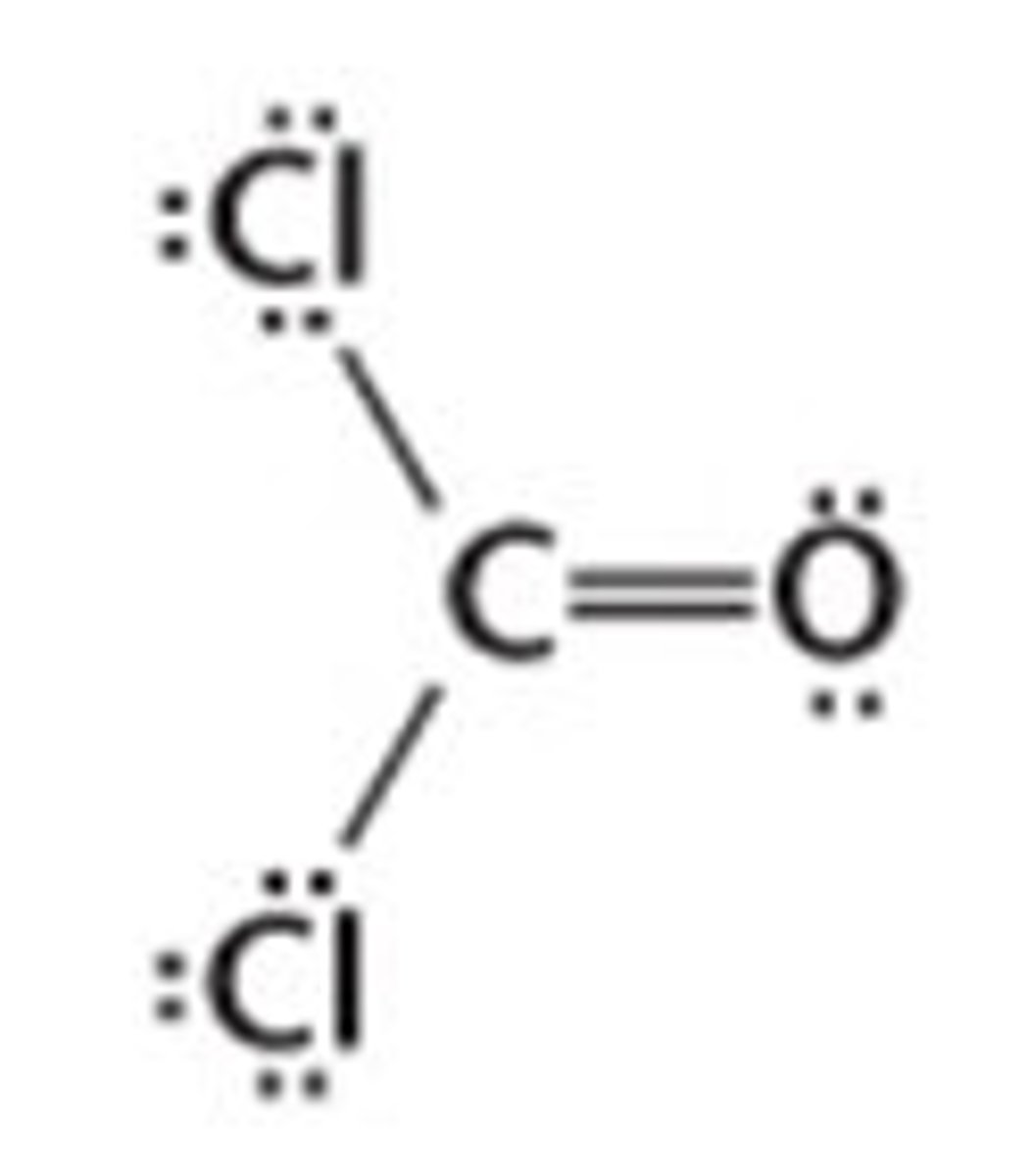
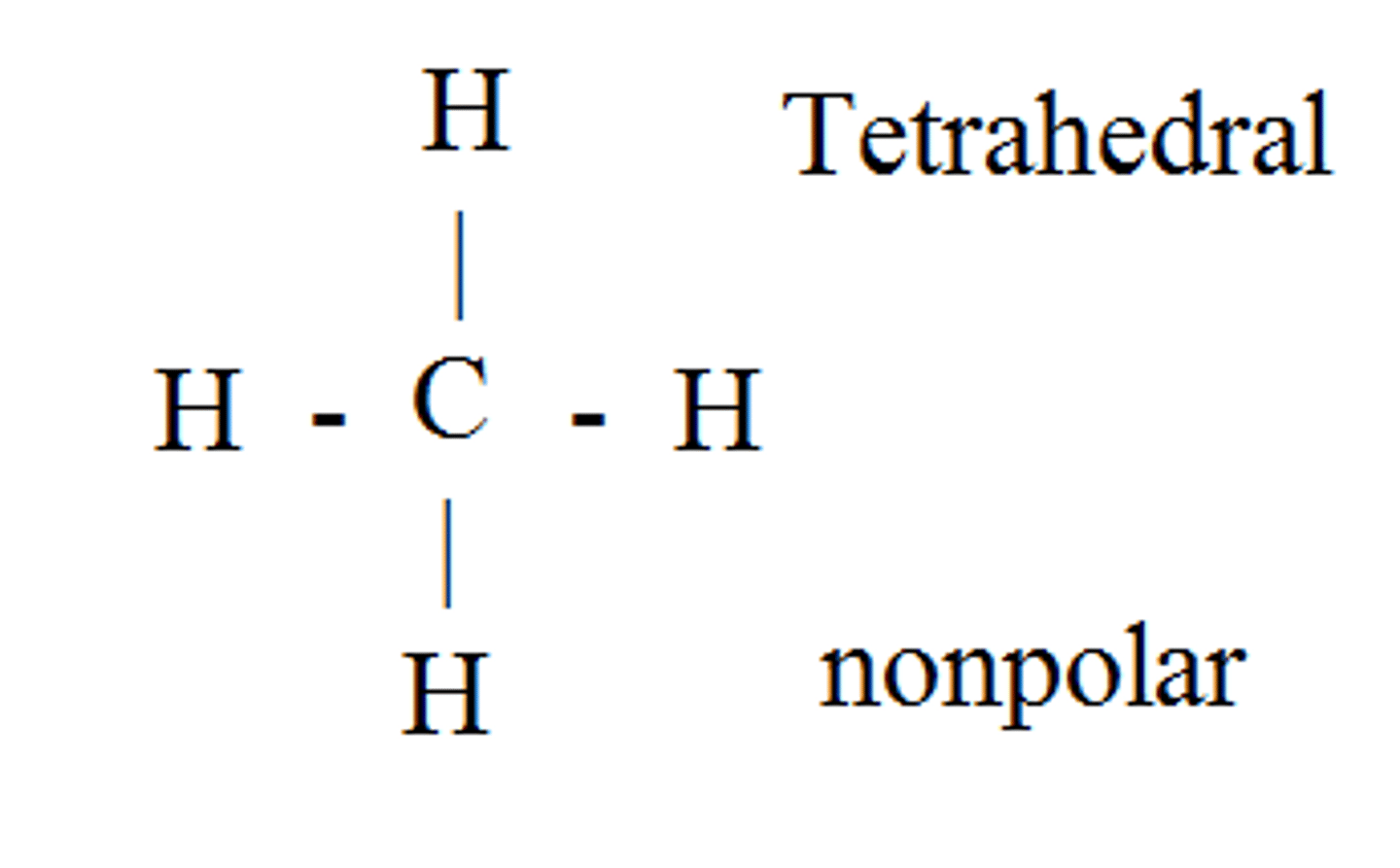
CCl₂O
trigonal planar, polar
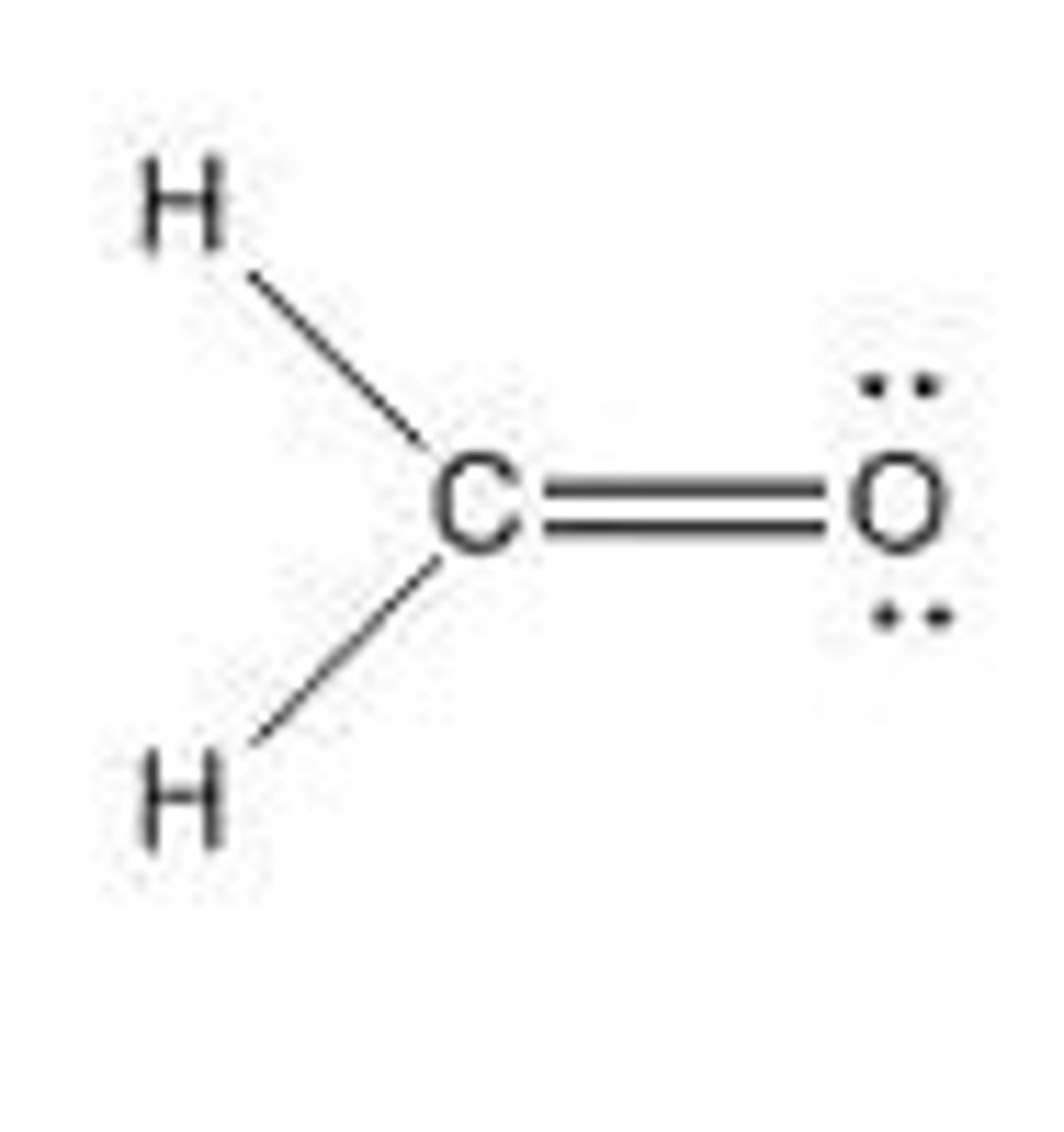
CH₂O
trigonal planar, polar
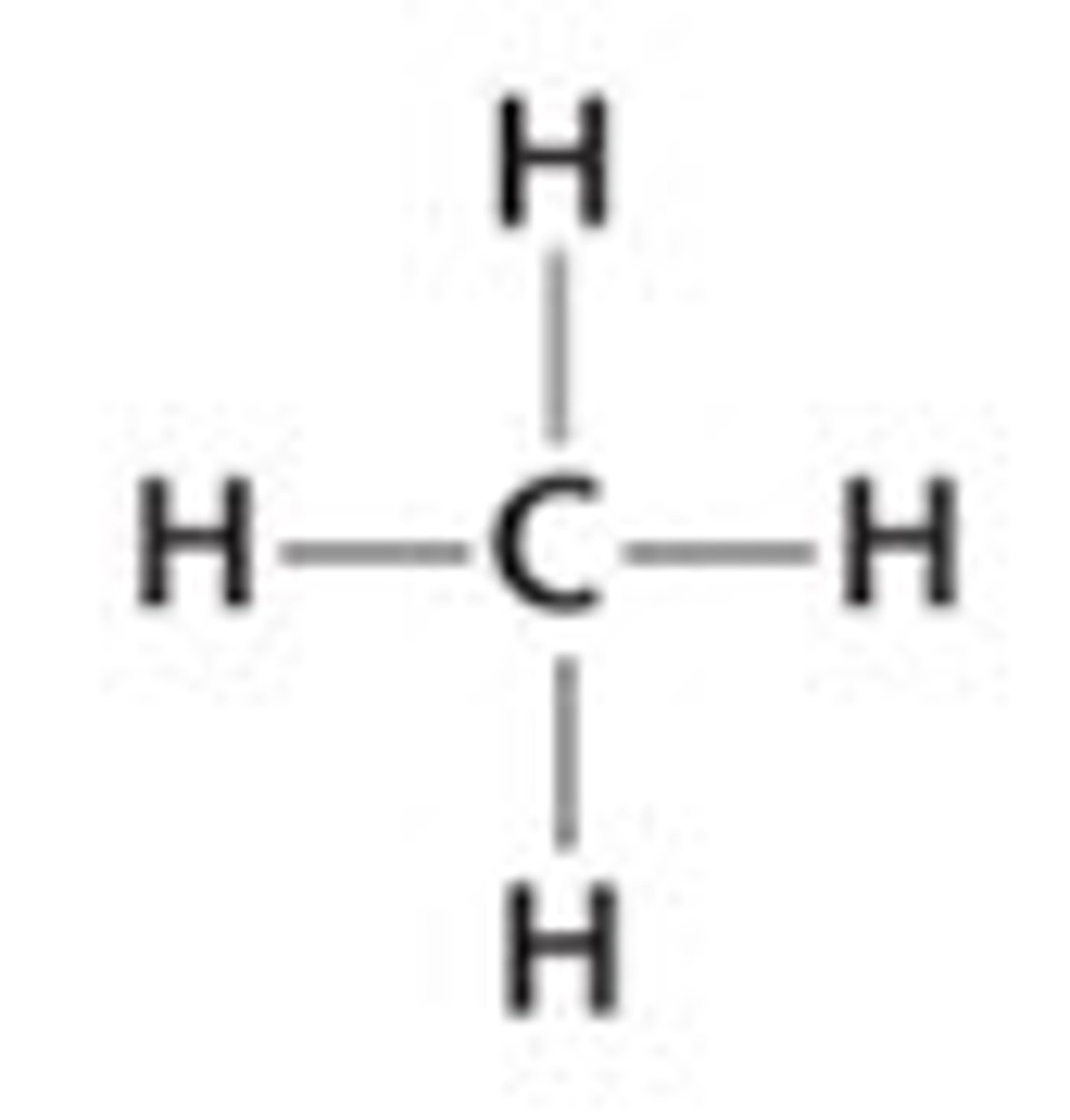
CH₄
tetrahedral, nonpolar
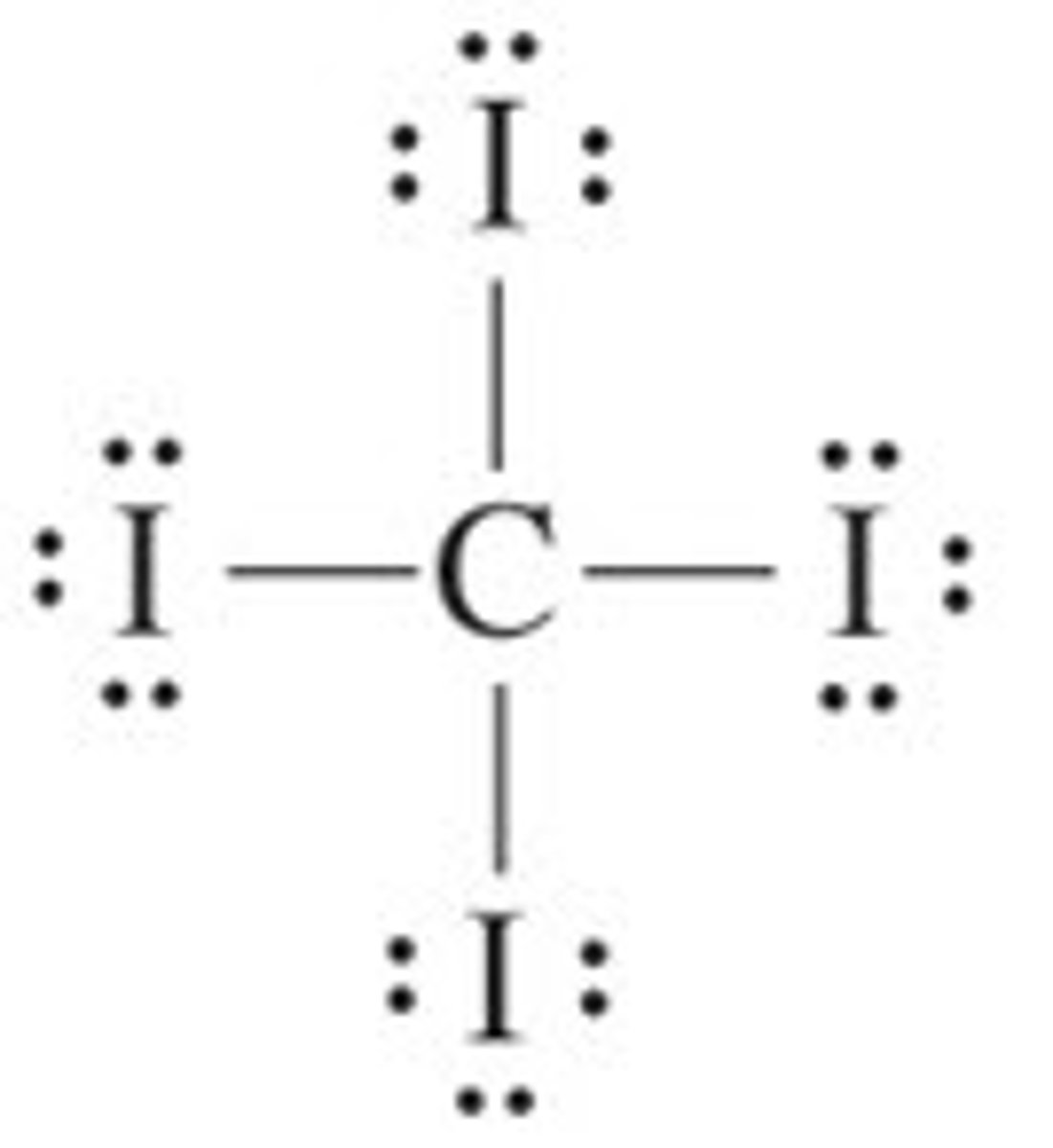
CI₄ (1-carbon, 4-iodine)
tetrahedral, nonpolar
Cl₂
linear, nonpolar
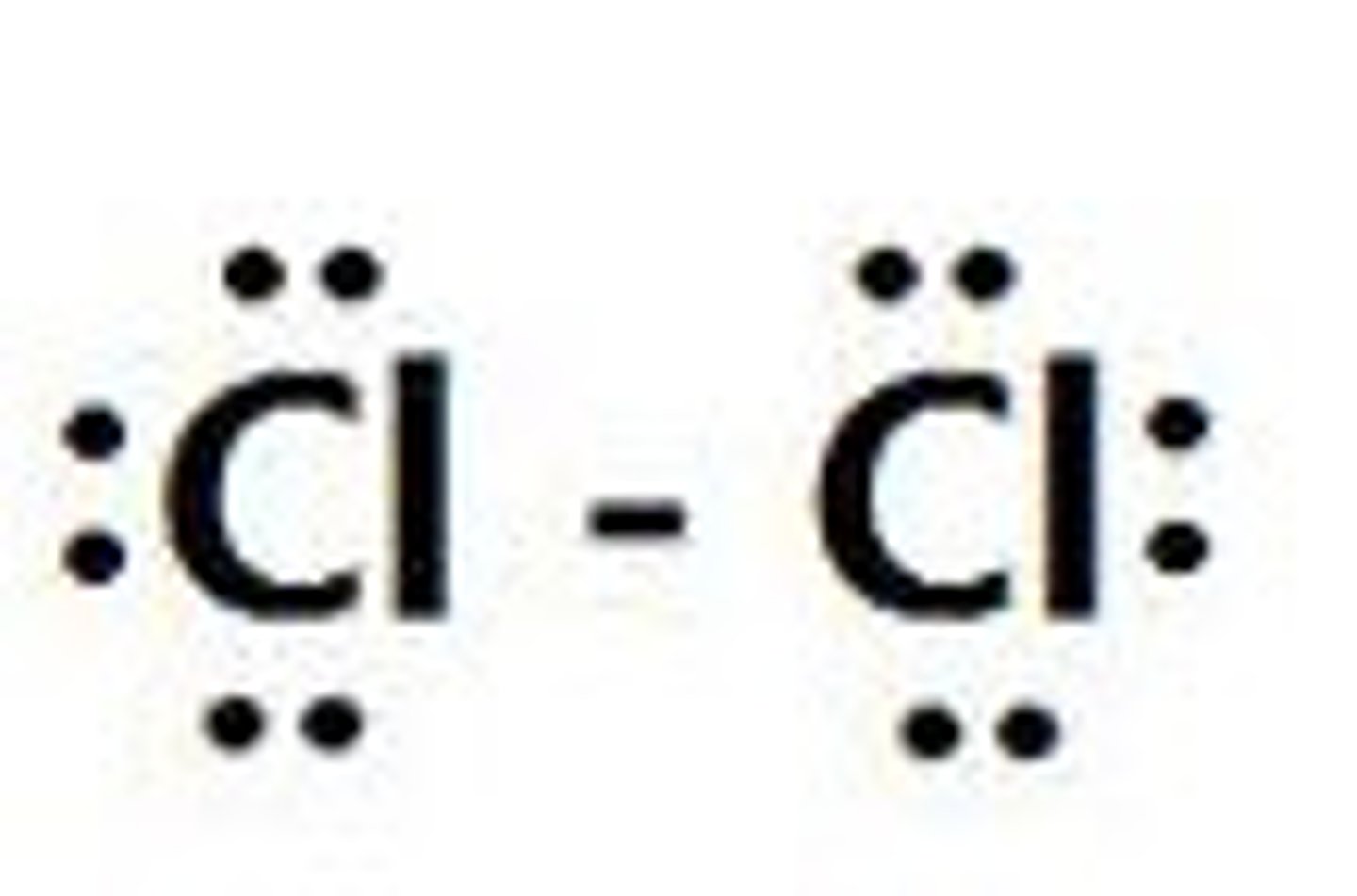
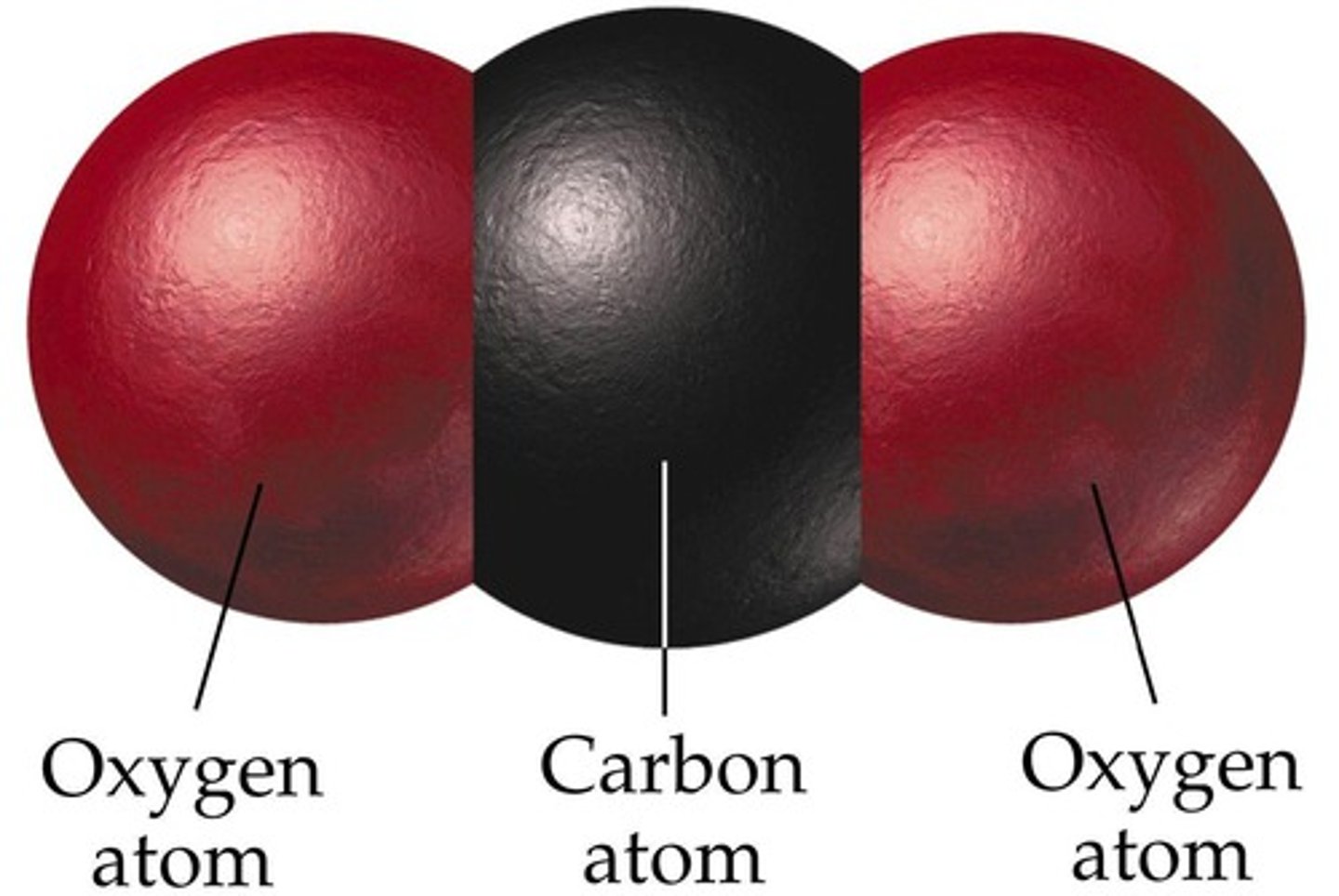
CO₂
linear, nonpolar
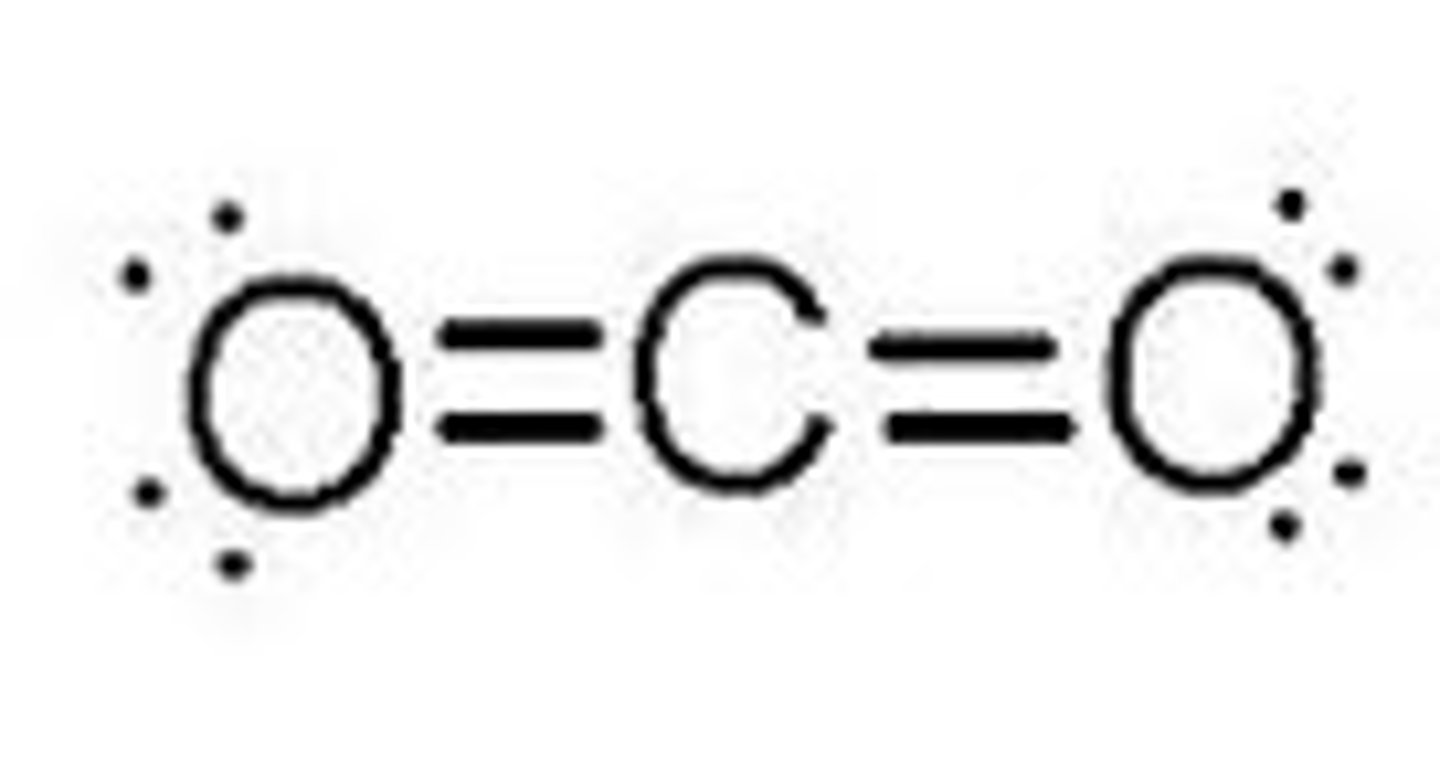
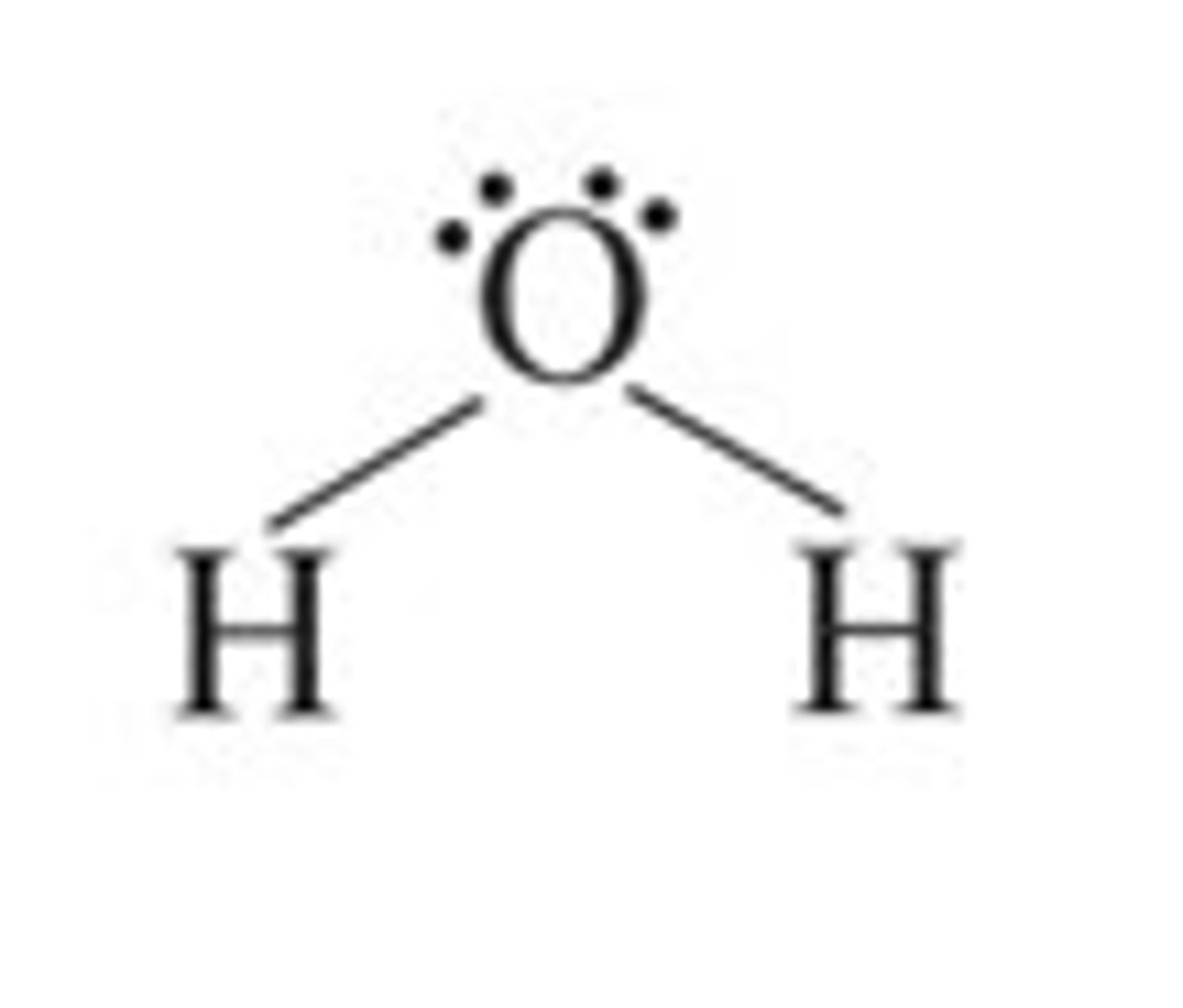
H₂O
bent, polar
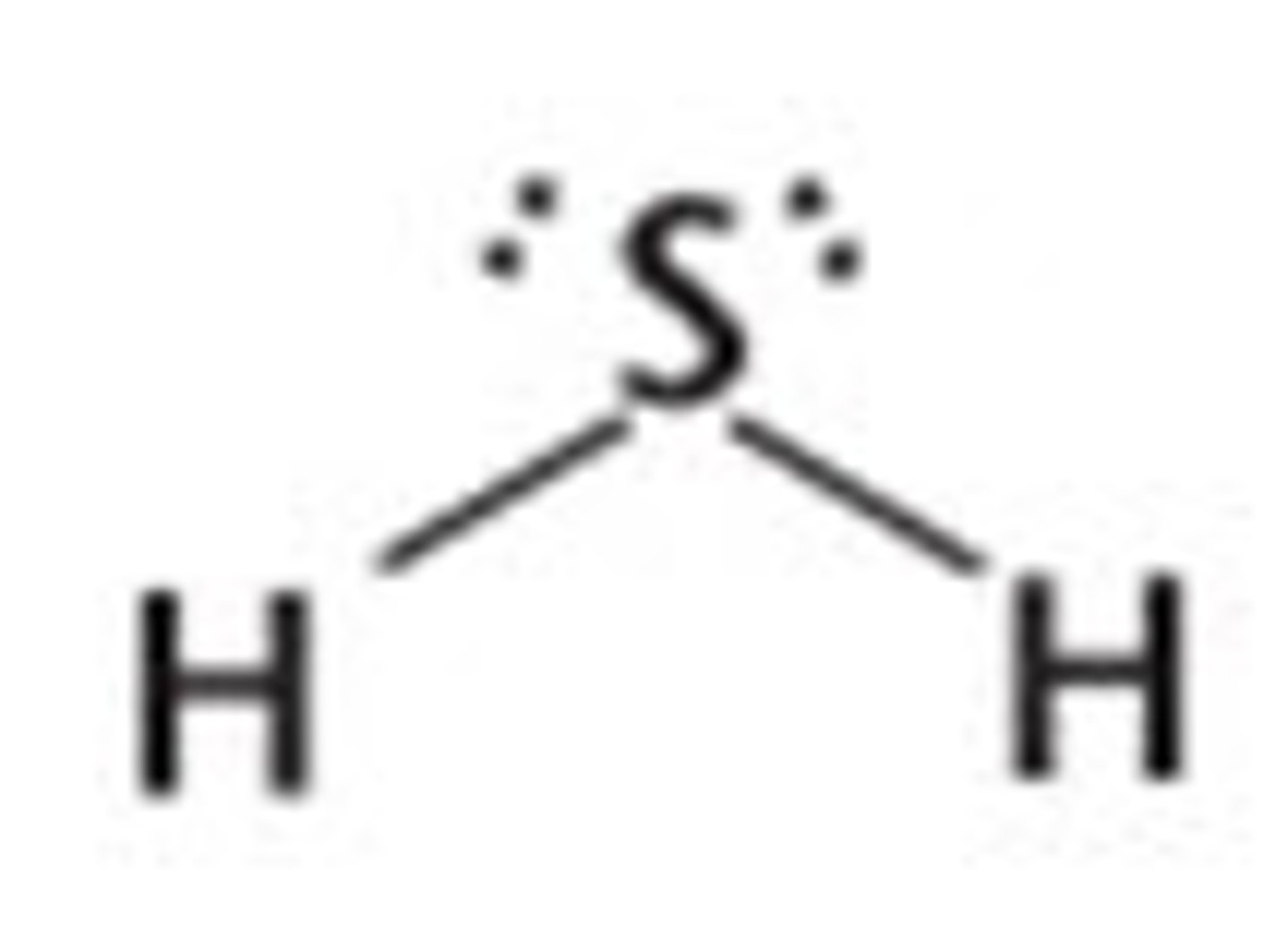
H₂S
bent, polar
HCl
linear, polar
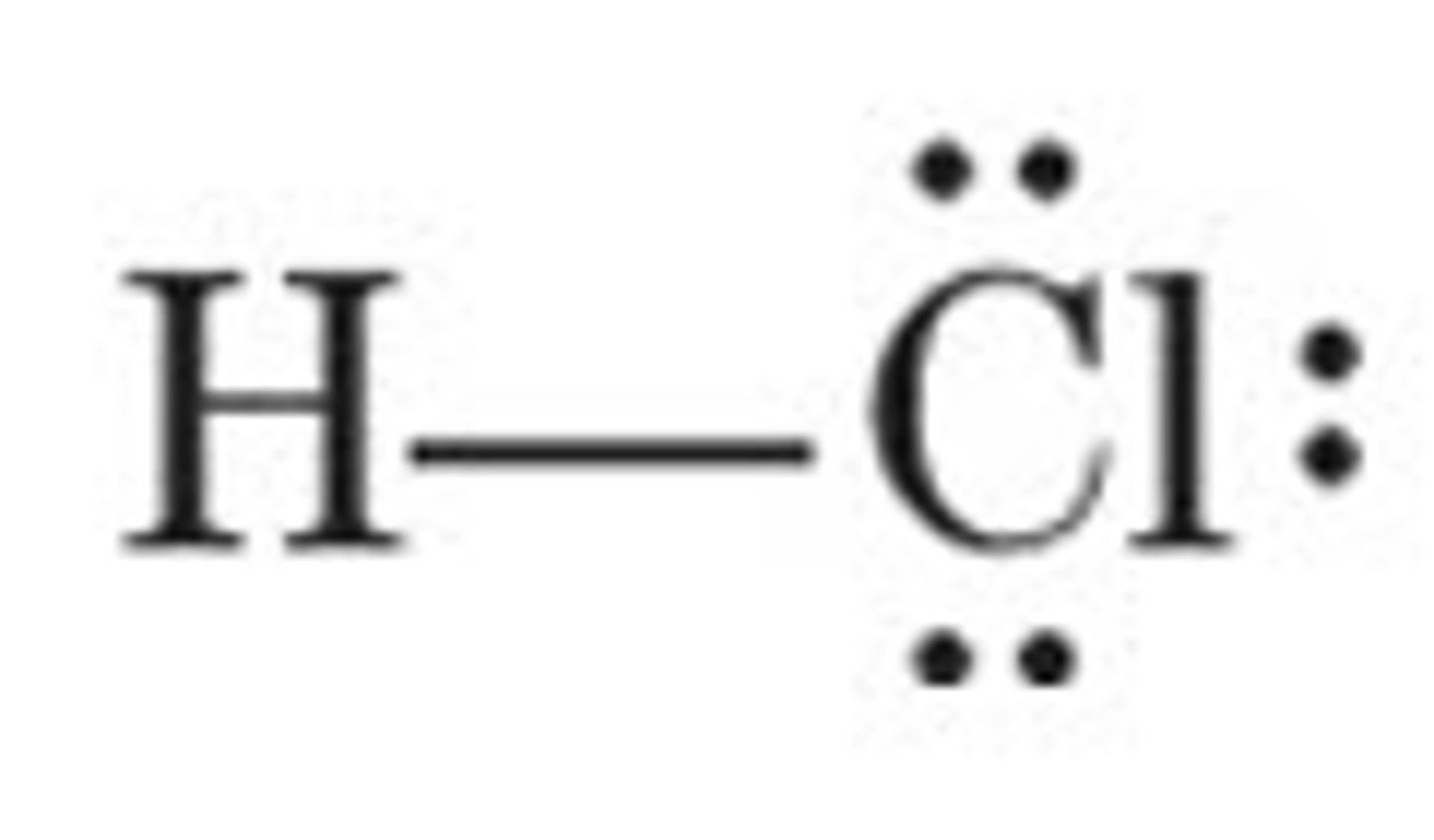
HCN
linear, polar

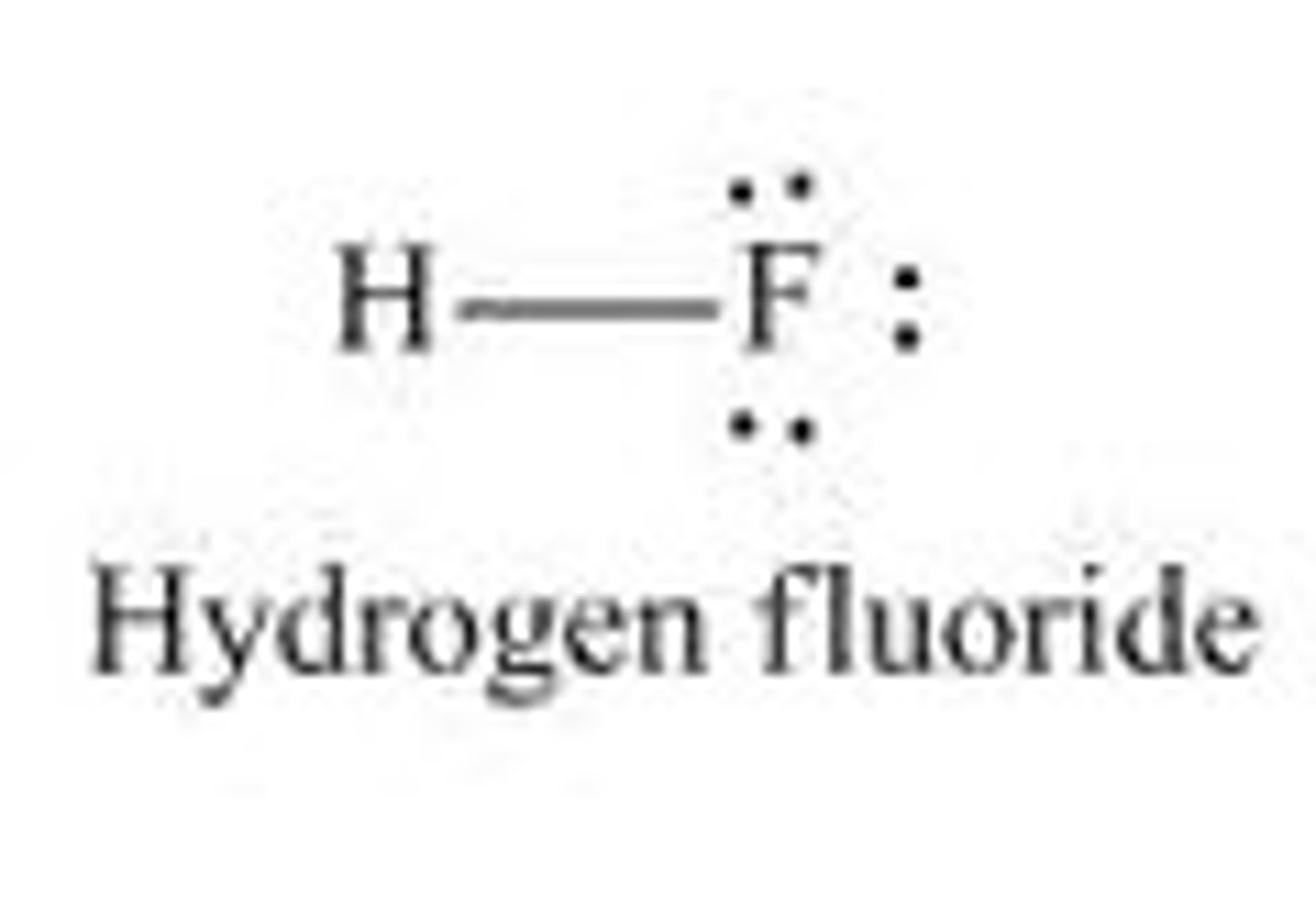
HF
linear, polar
N₂
linear, nonpolar
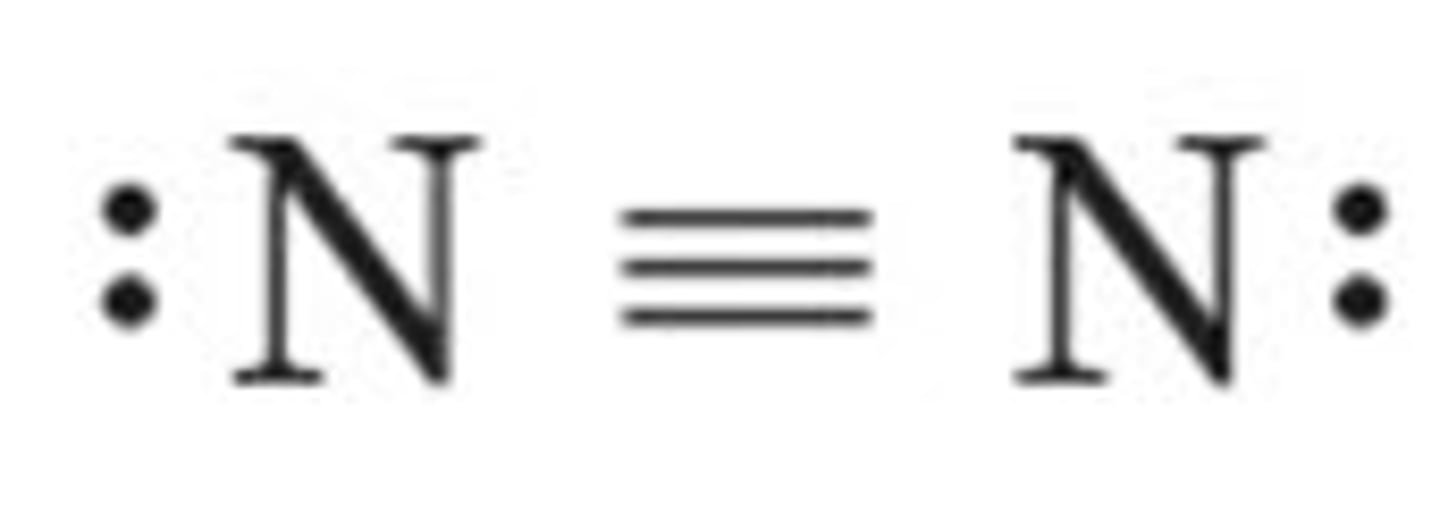
NH₃
pyramidal, polar
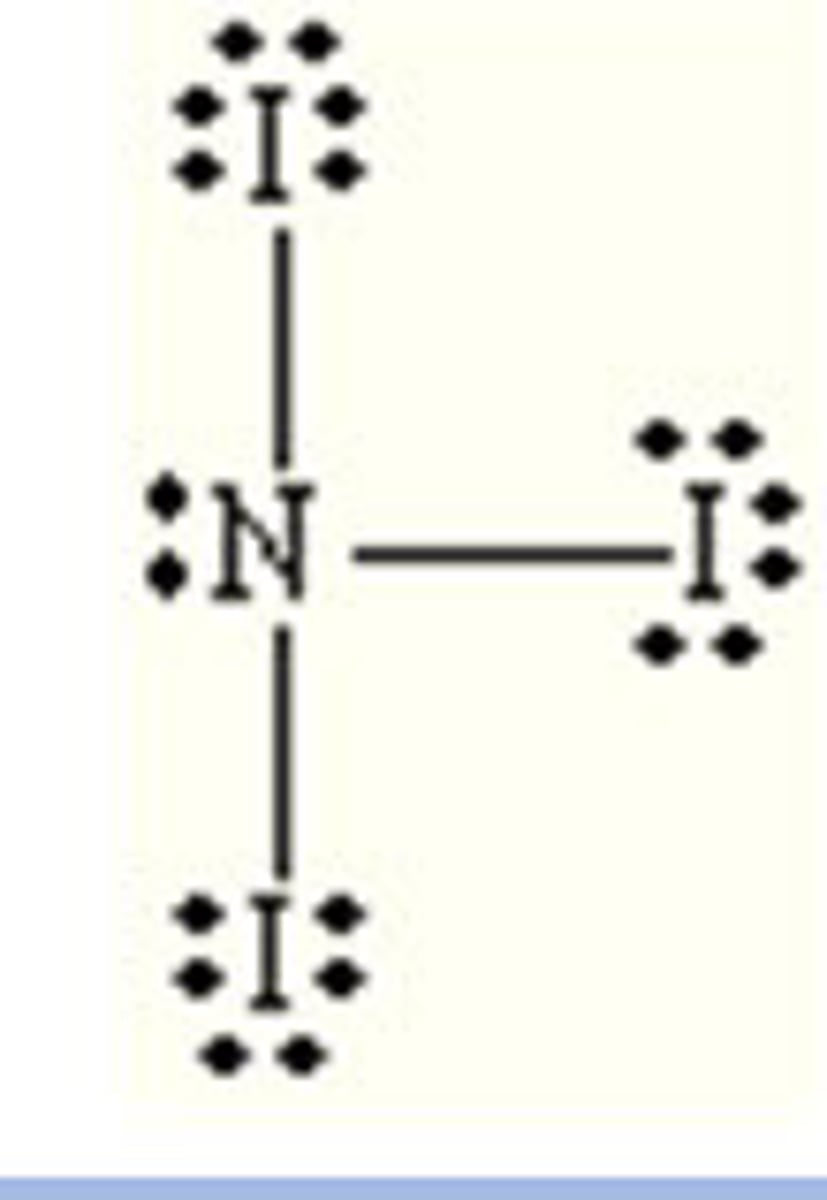
NI₃
pyramidal, polar
PCl₃
pyramidal, polar
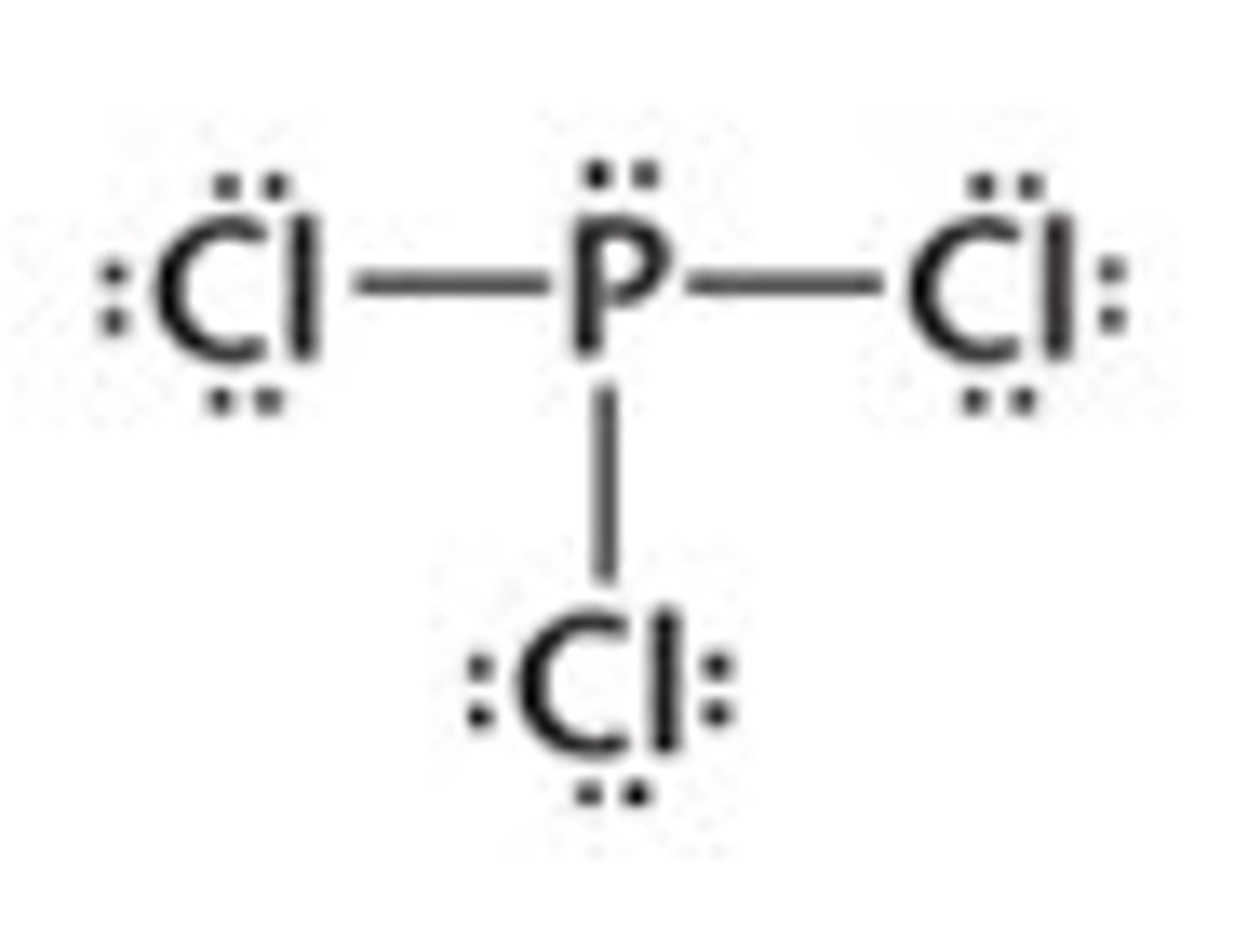
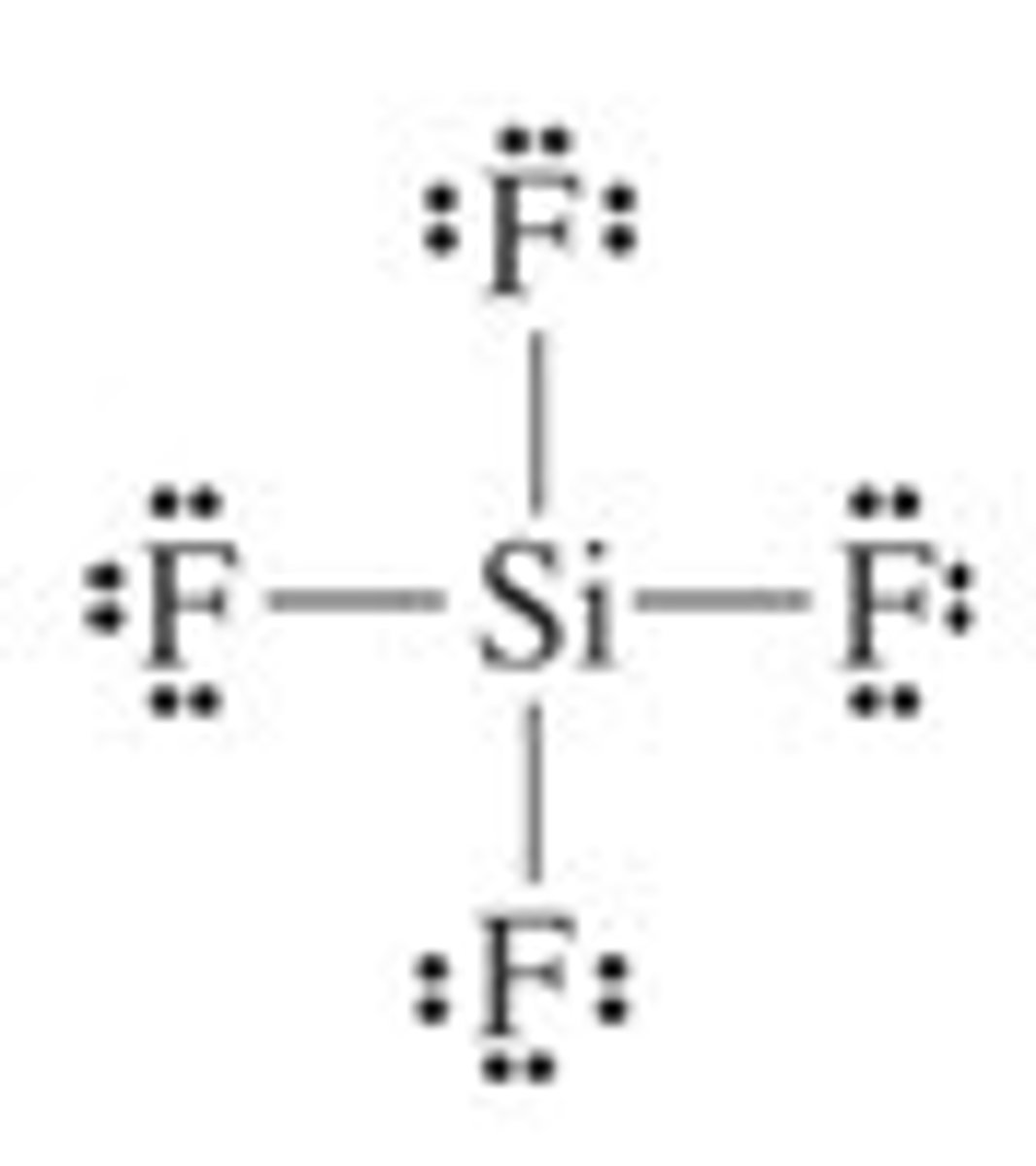
SiF₄
tetrahedral, nonpolar
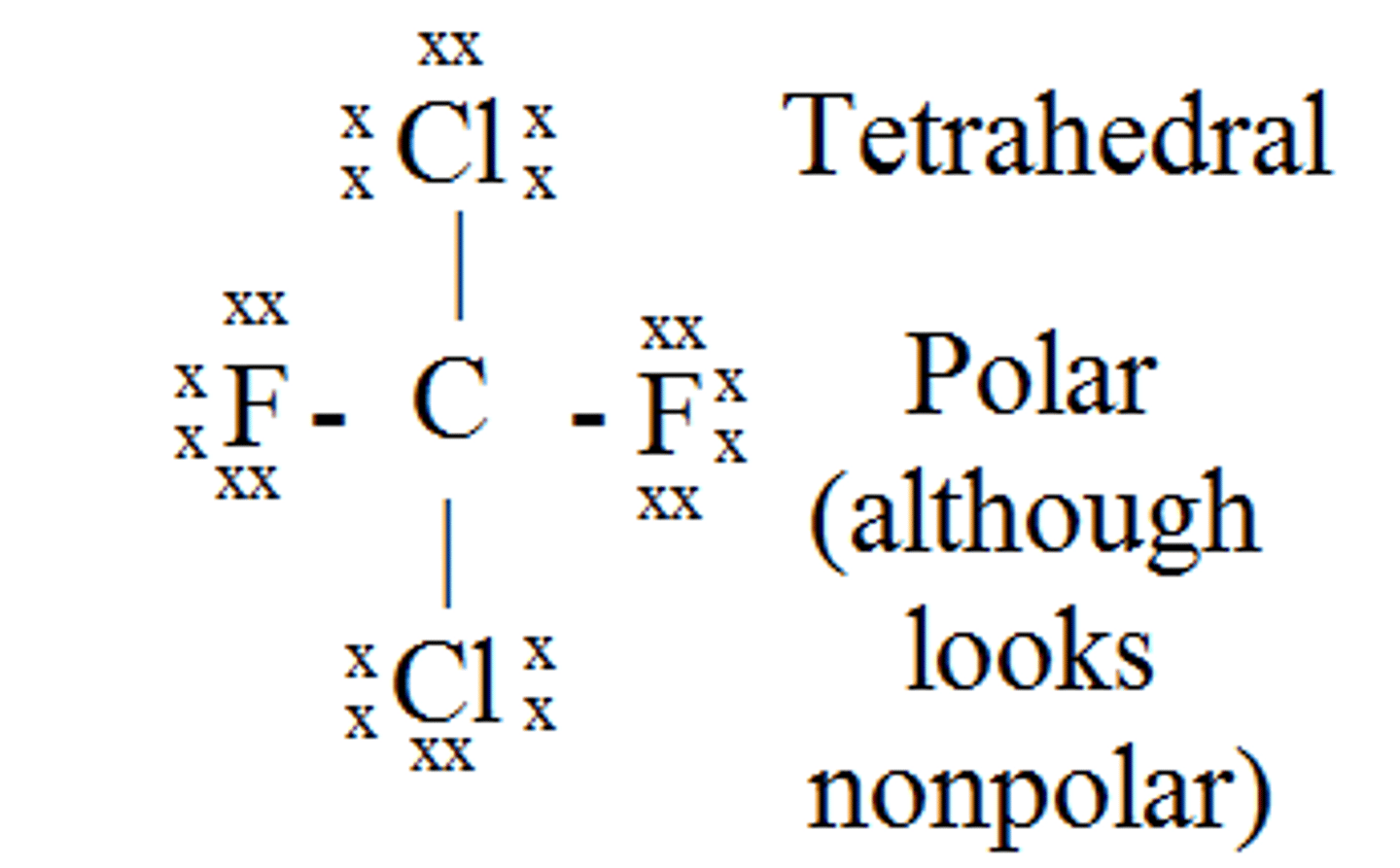
SO₃
trigonal planar, polar

H₂O
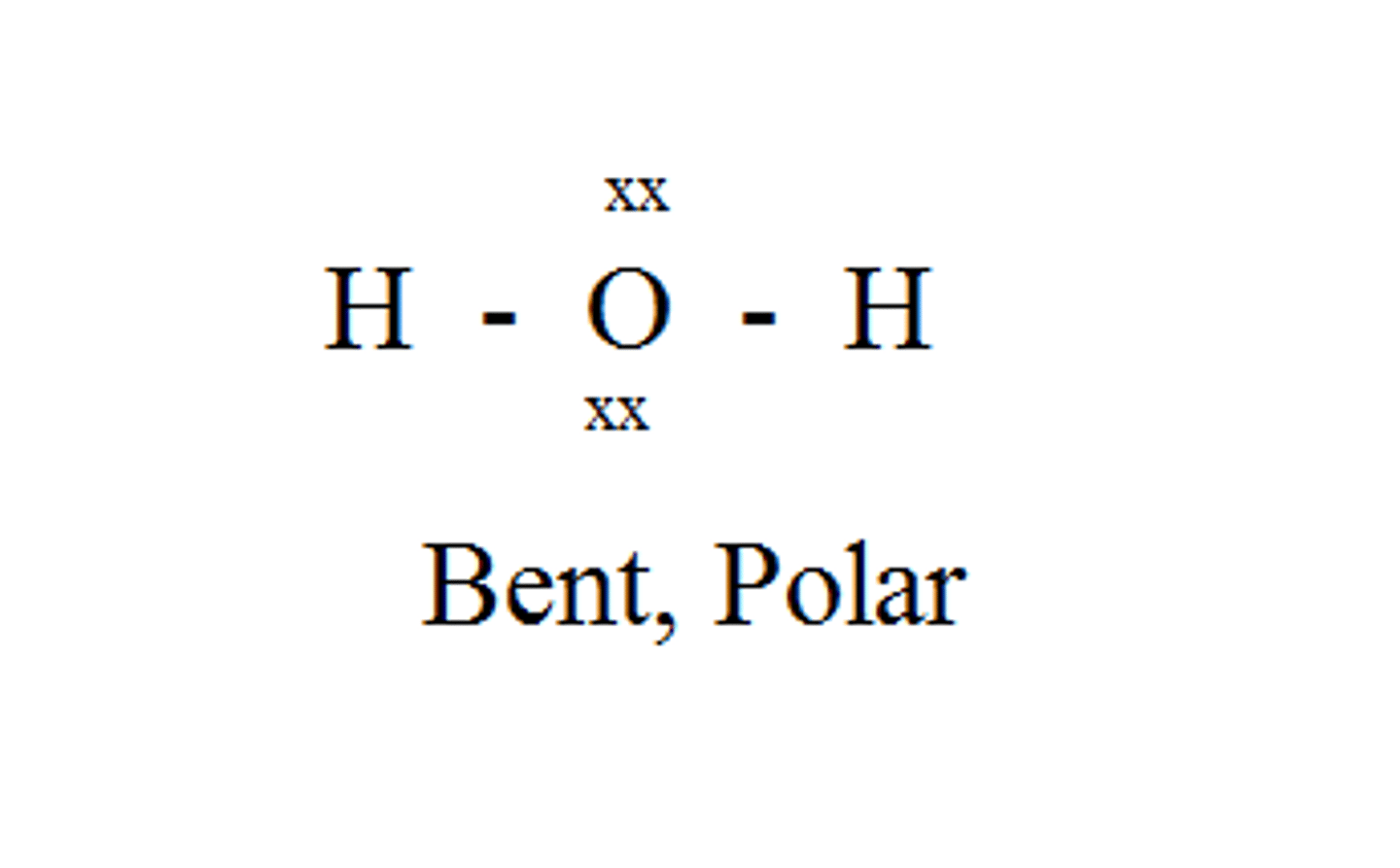
NH₃
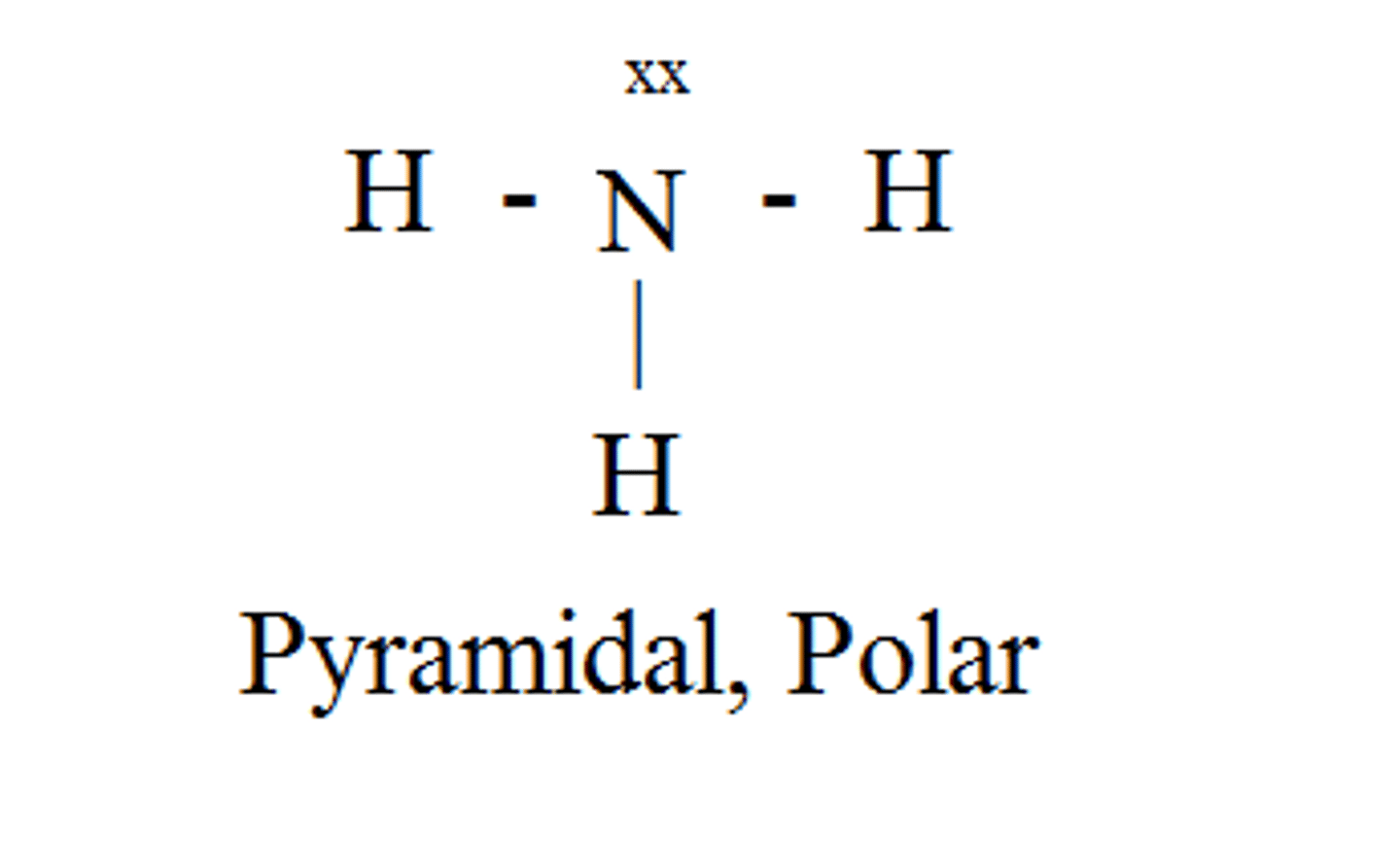
CH₄
CF₂Cl₂
SO₃
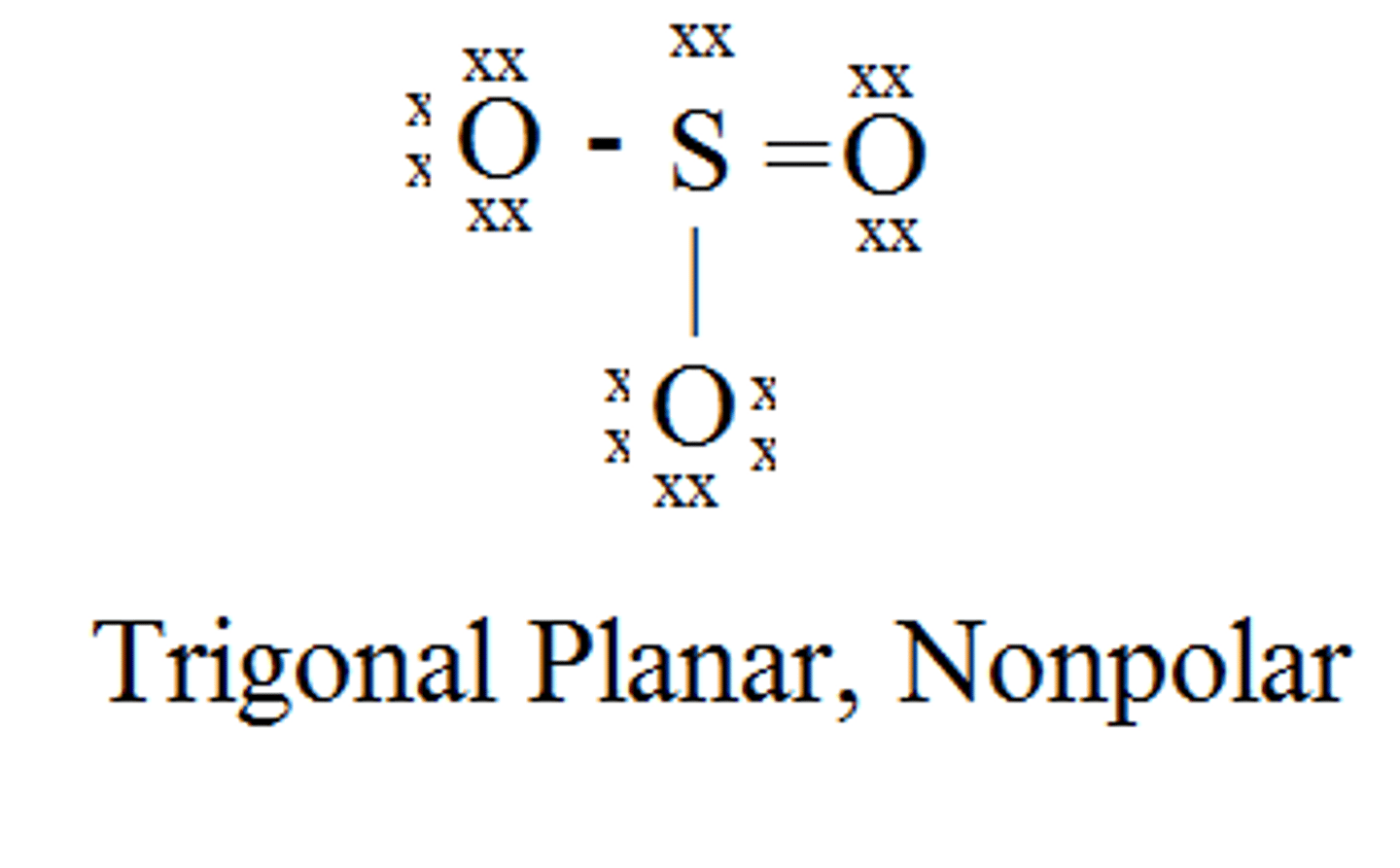
O₃
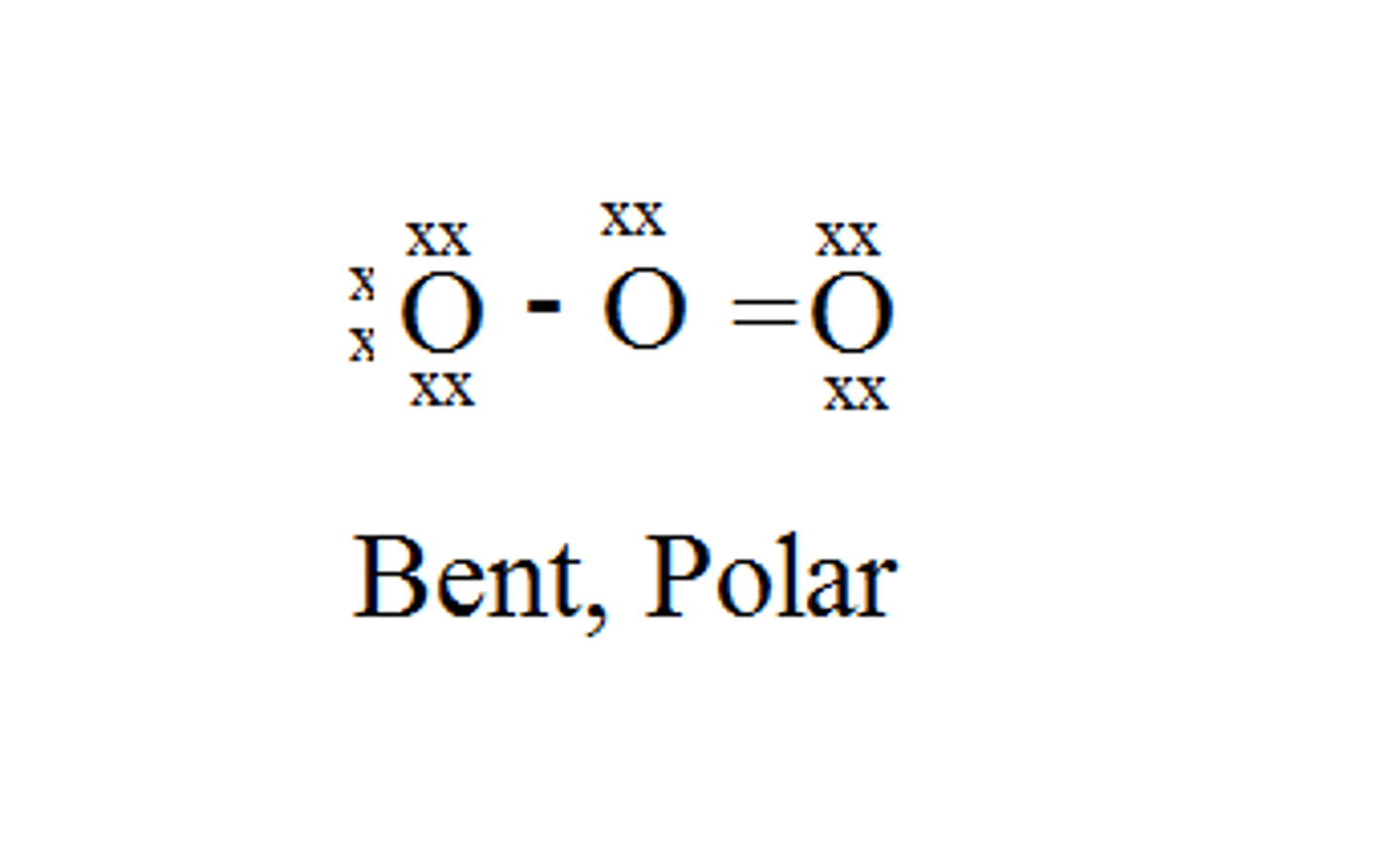
SO₂
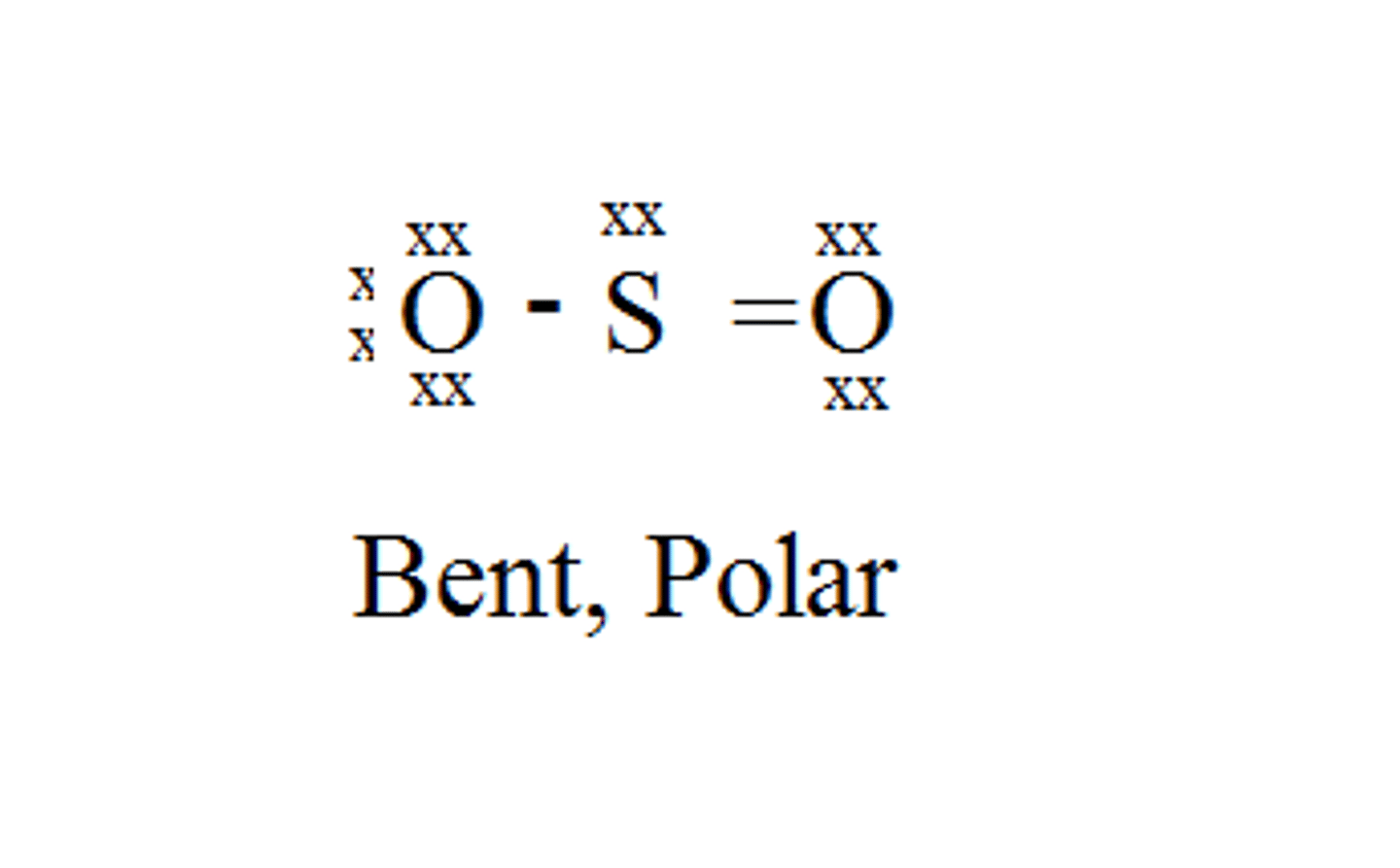
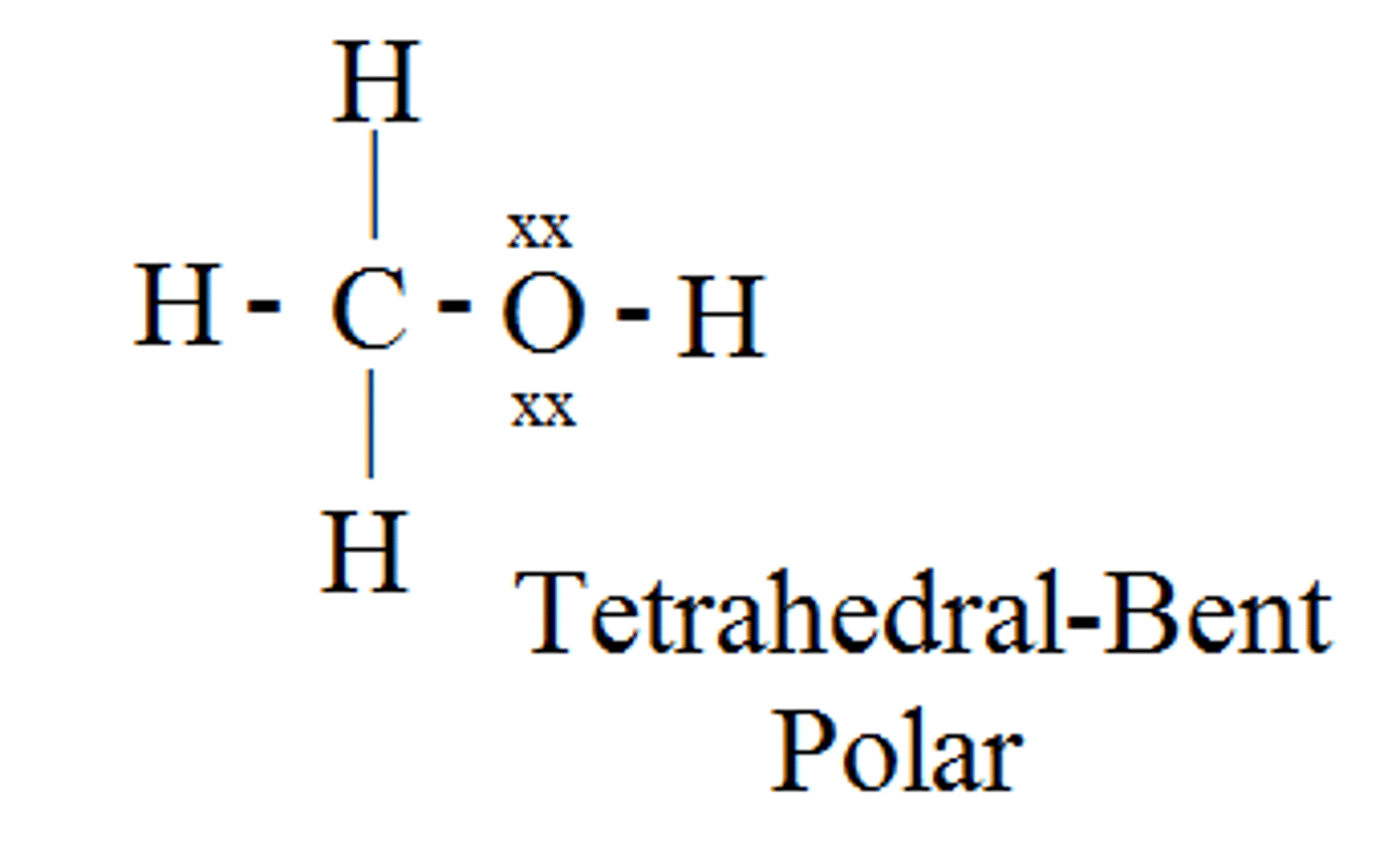
CH₃OH
*Ionic Bonding
Occurs when electrons are transferred between
atoms. Formed by the attraction between a metal and
nonmetal
Cation
Formed when the metal donates one or more electrons to the nonmetal
Anion
Formed when a nonmetal accepts one or more electrons
*"CO"valent Bonding
Occurs when pairs of electrons are shared between atoms.When atoms have different electronegativities, these
electrons are shared "unfairly".
Types of bonds:
- Single: 2 electrons shared
- Double: 4 electrons shared
- Triple: 6 electrons shared
Octet Rule
Elements prefer to have eight valence electrons in their outer
shells. Hydrogen and Helium are exceptions.
Ionic Bonding
Transfer of electrons from one atom to another
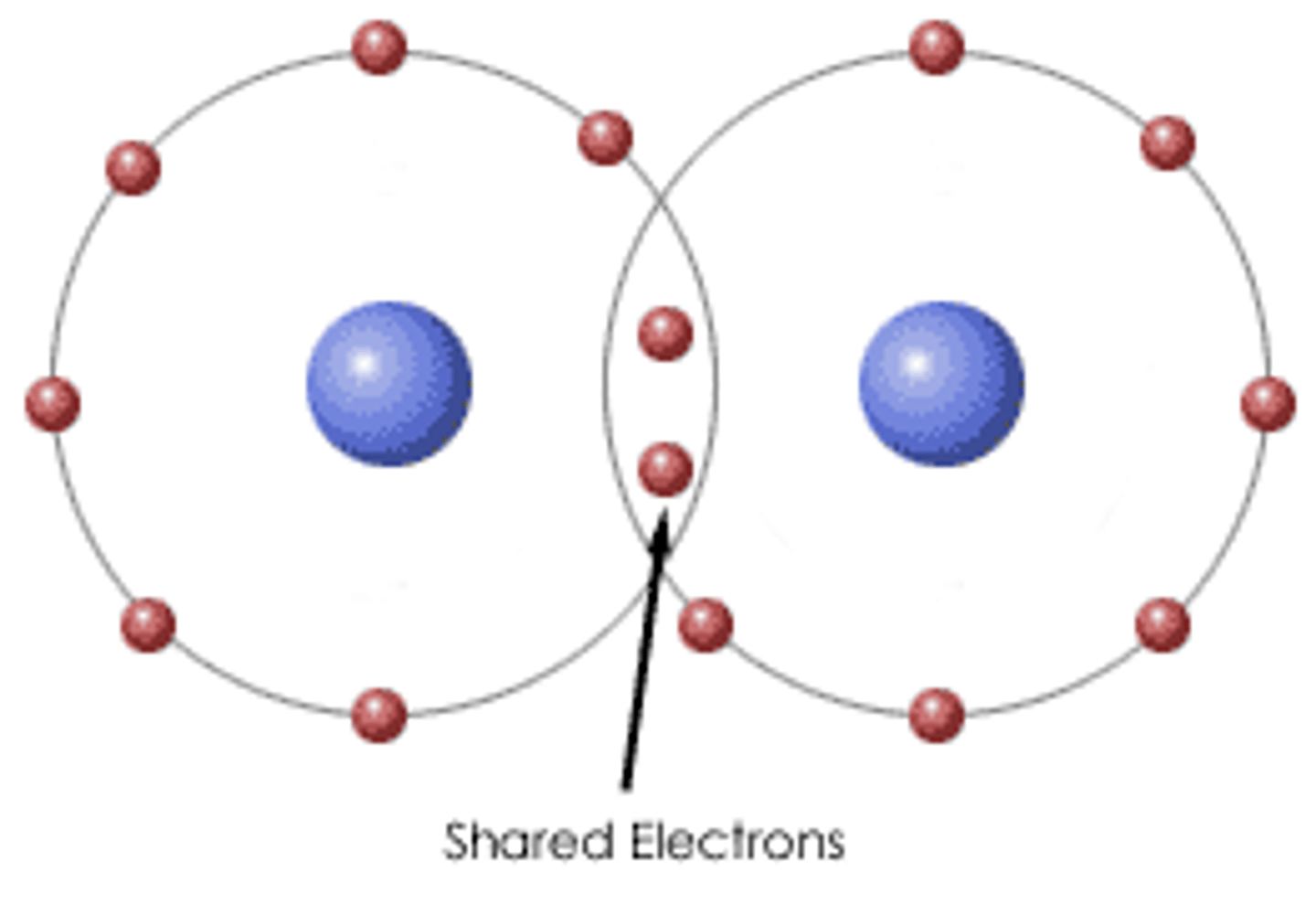
Covalent Bonding
sharing of
electrons between two atoms
Steps for writing Lewis Structures
1. Add up the valence electrons for all atoms in
the compound. (Be careful to add or subtract
electrons for ions)
2. Place the least electronegative element at
the center (except for hydrogen)
3. Add SINGLE covalent bonds to connect the
atoms.
4. Add lone (unshared) pairs to the outer atoms.
5. Add lone pairs to the center atom.
6. Check all atoms for octet rule (having 8
electrons, except for H and He). If necessary,
move electrons to create double or triple bonds.
7. If applicable, draw the charge of the ion. For a
polyatomic ion, put the whole Lewis structure in
brackets first.
A lone pair is any 2 electrons not in a covalent bond
Sulfate ion
• SO42̶
• 6 + (6 x 4) + 2= 32 valence electrons
Ammonium ion
• NH4+
• 5 + (1 x 4) - 1 = 8 valence electrons
Carbon
• C
• 4 valence electrons
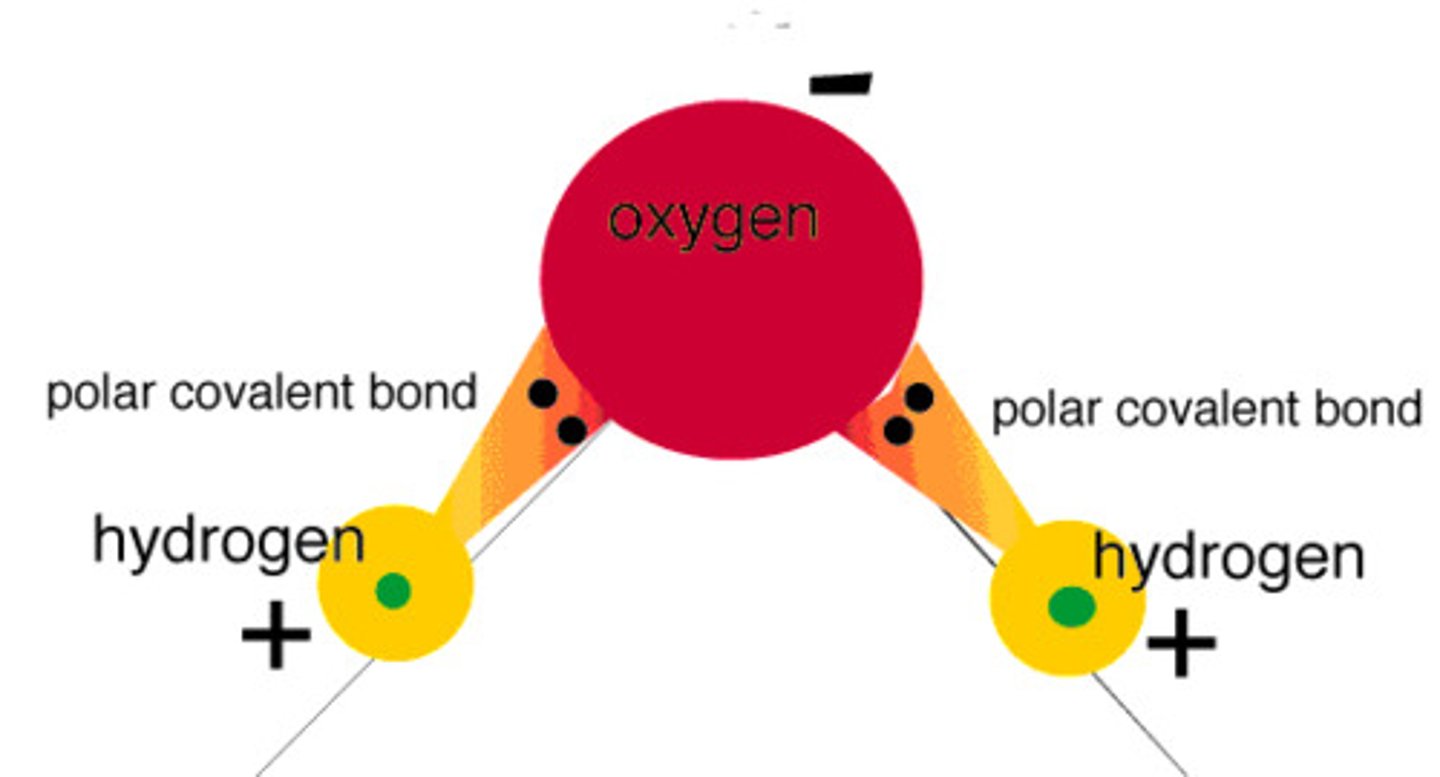
bond moment (dipole)
a vector that indicates the size of the polarity of a particular covalent bond (within a molecule)- points in the direction of the more electronegative atom
(in the eg- The C−H and C=C bonds are non-polar bonds, so have no bond moments associated with them)
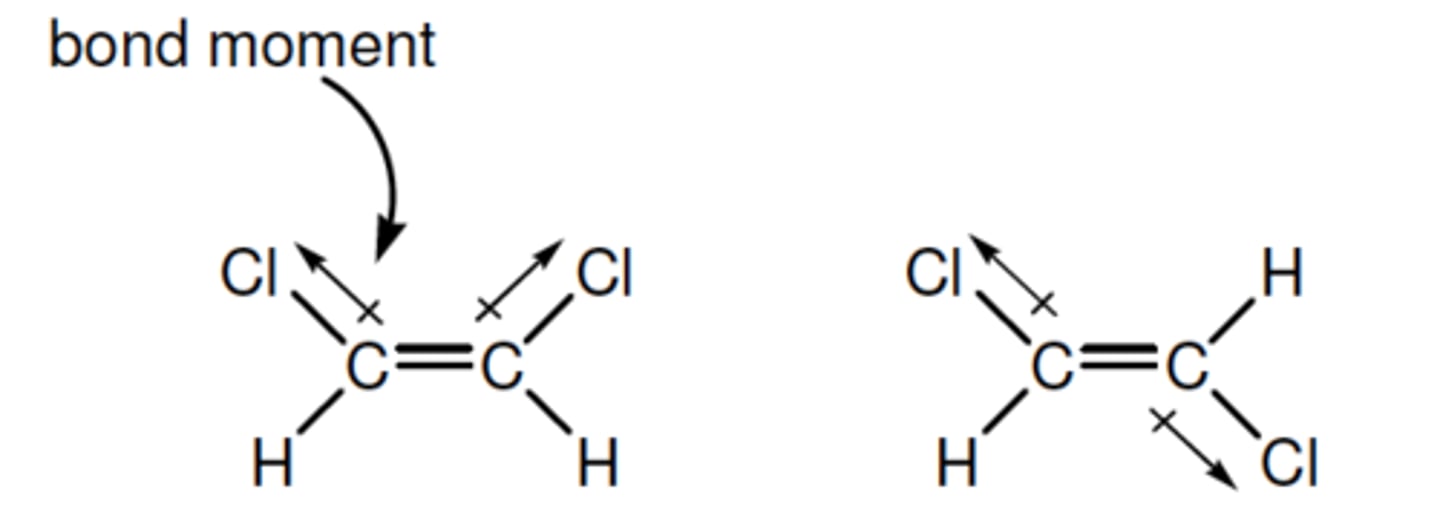
dipole moment
sum of individual bond moments. If the molecule has an overall dipole moment it is polar- the moment will indicate its direction
EG- The molecule on the left had two bond moments
which, by vector addition, give the overall dipole
moment shown.
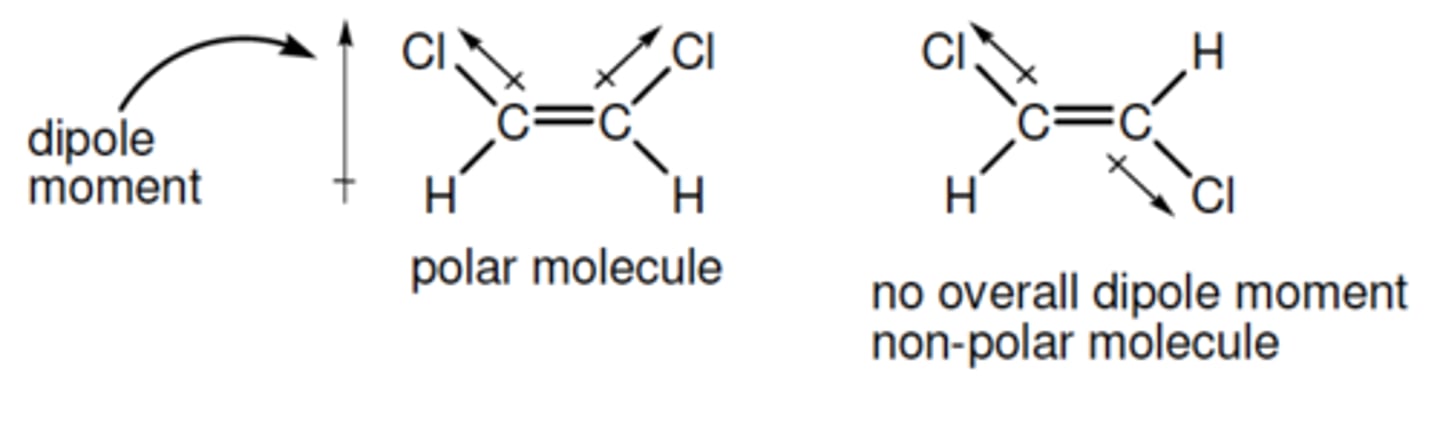
polar molecule
molecule that has an overall dipole moment
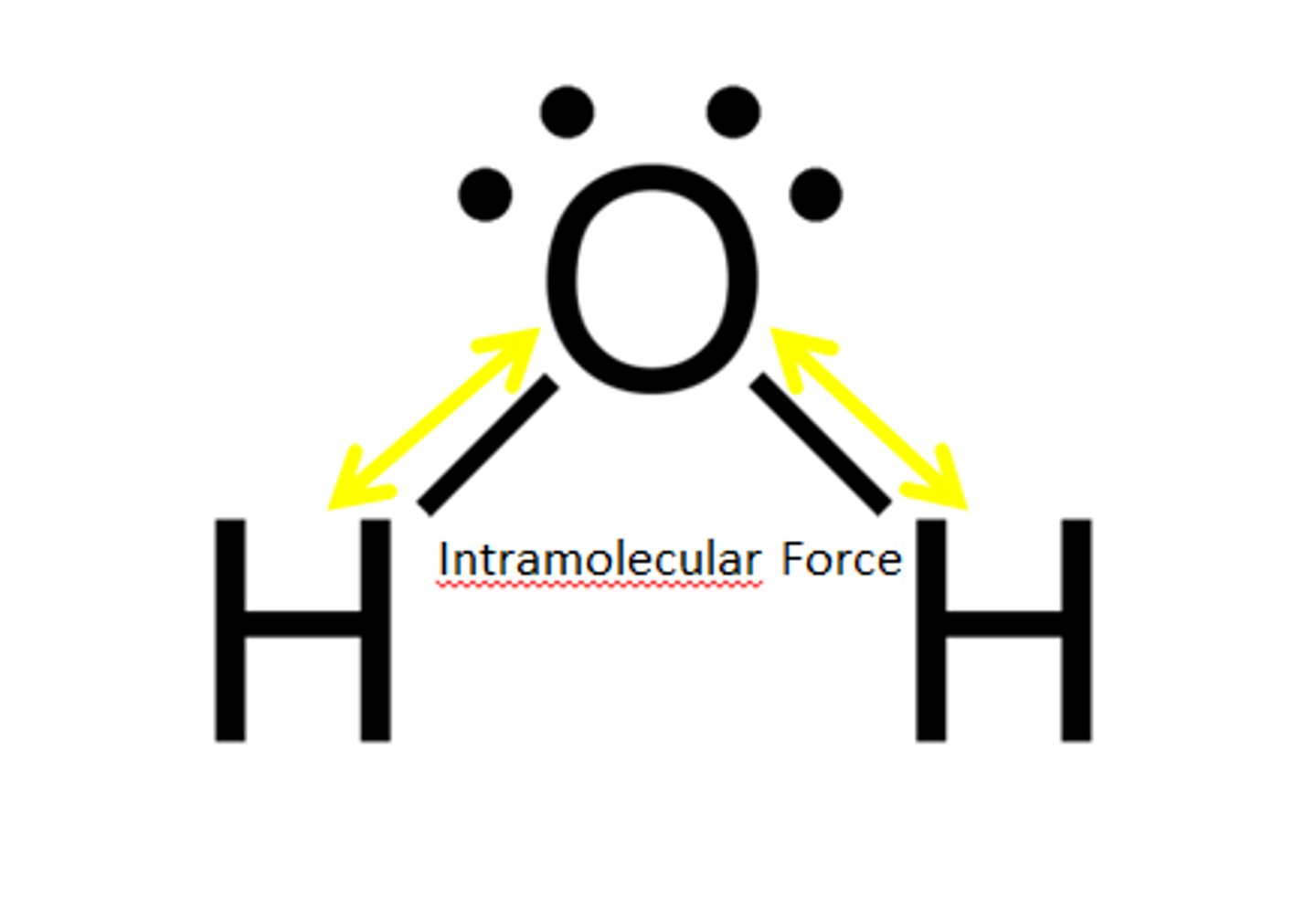
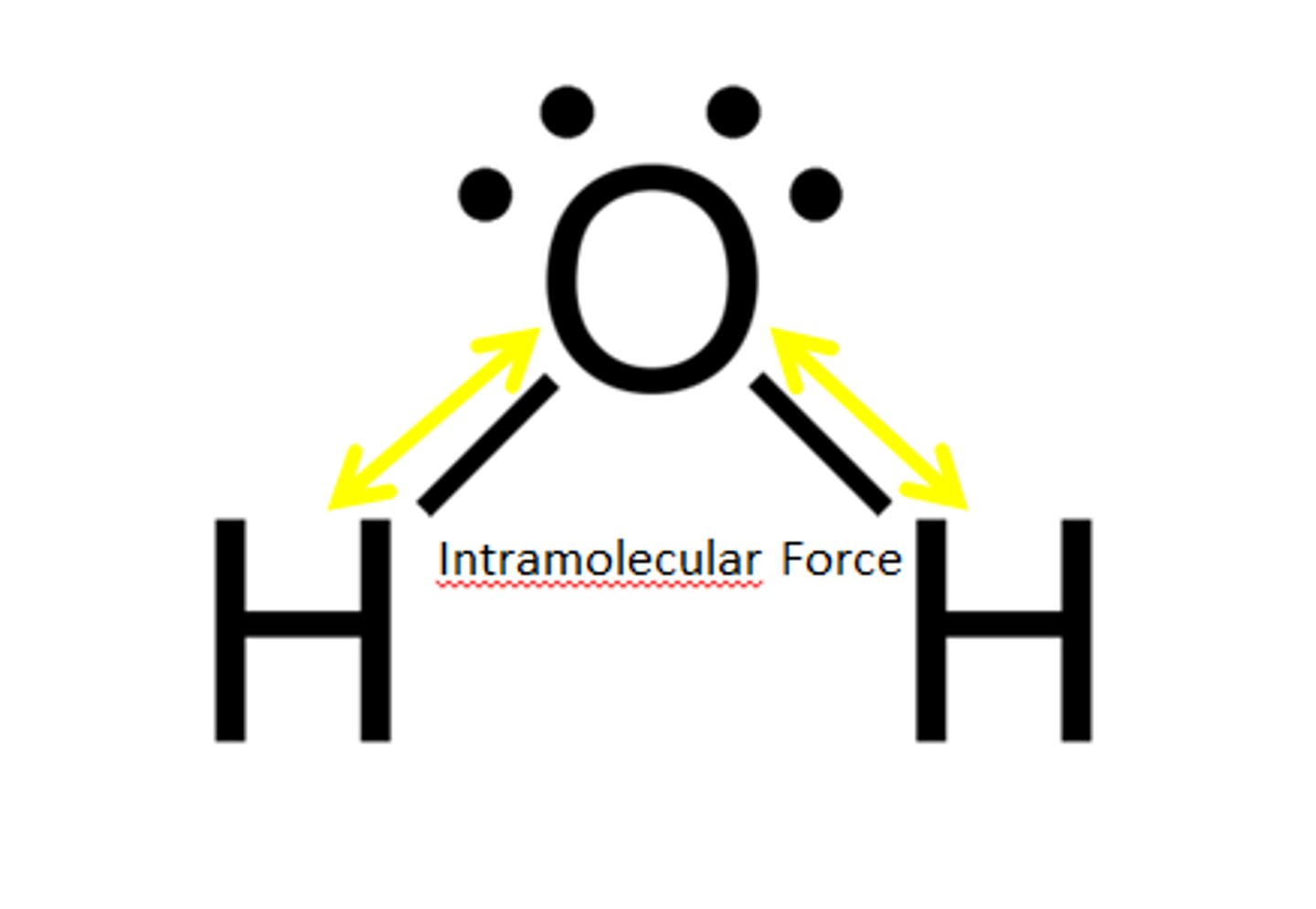
Intramolecular forces
forces that hold the atoms together in a molecule
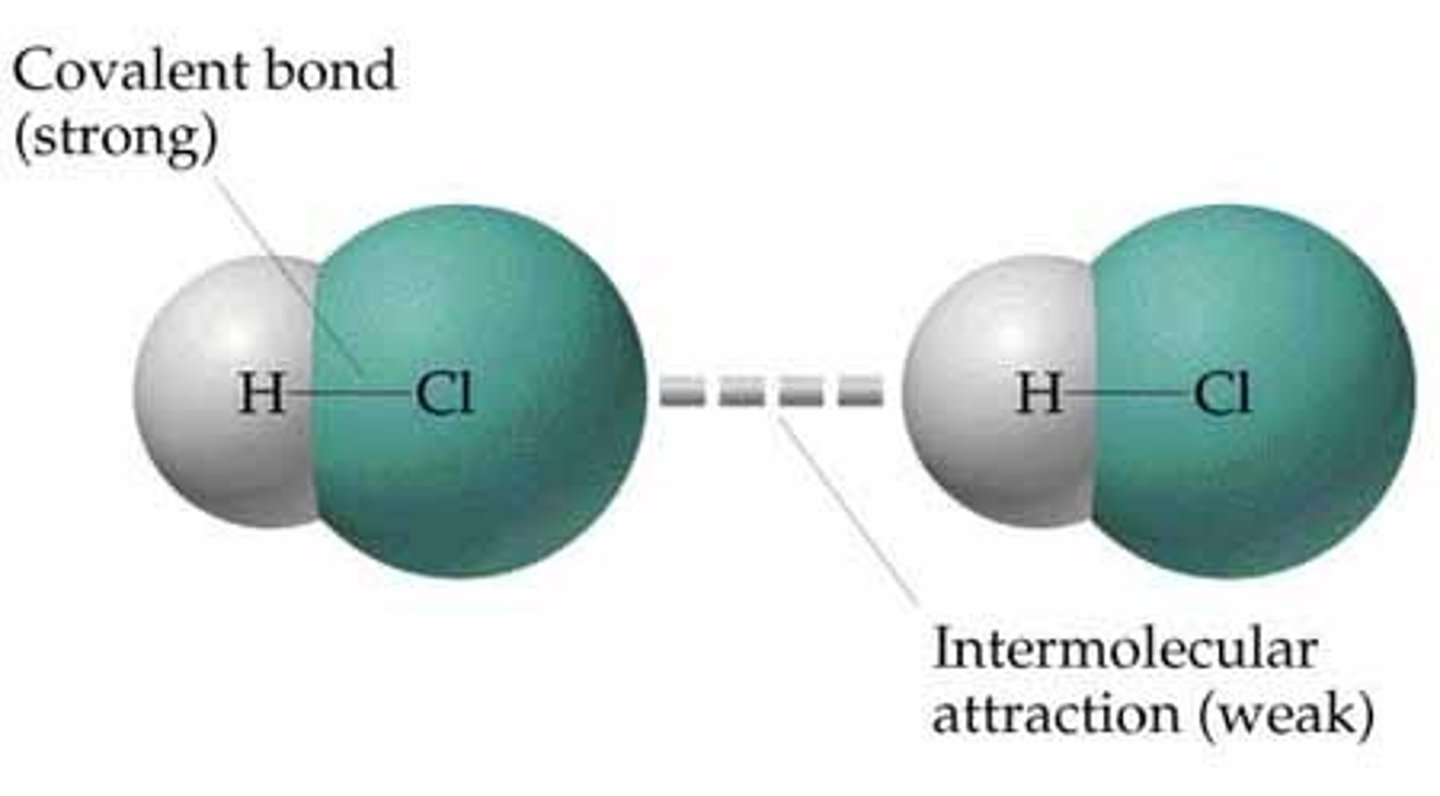
Intermolecular forces
interactions between molecules (much weaker)
intermolecular force strength
measures by: boiling and melting point- the higher, the stronger the forces
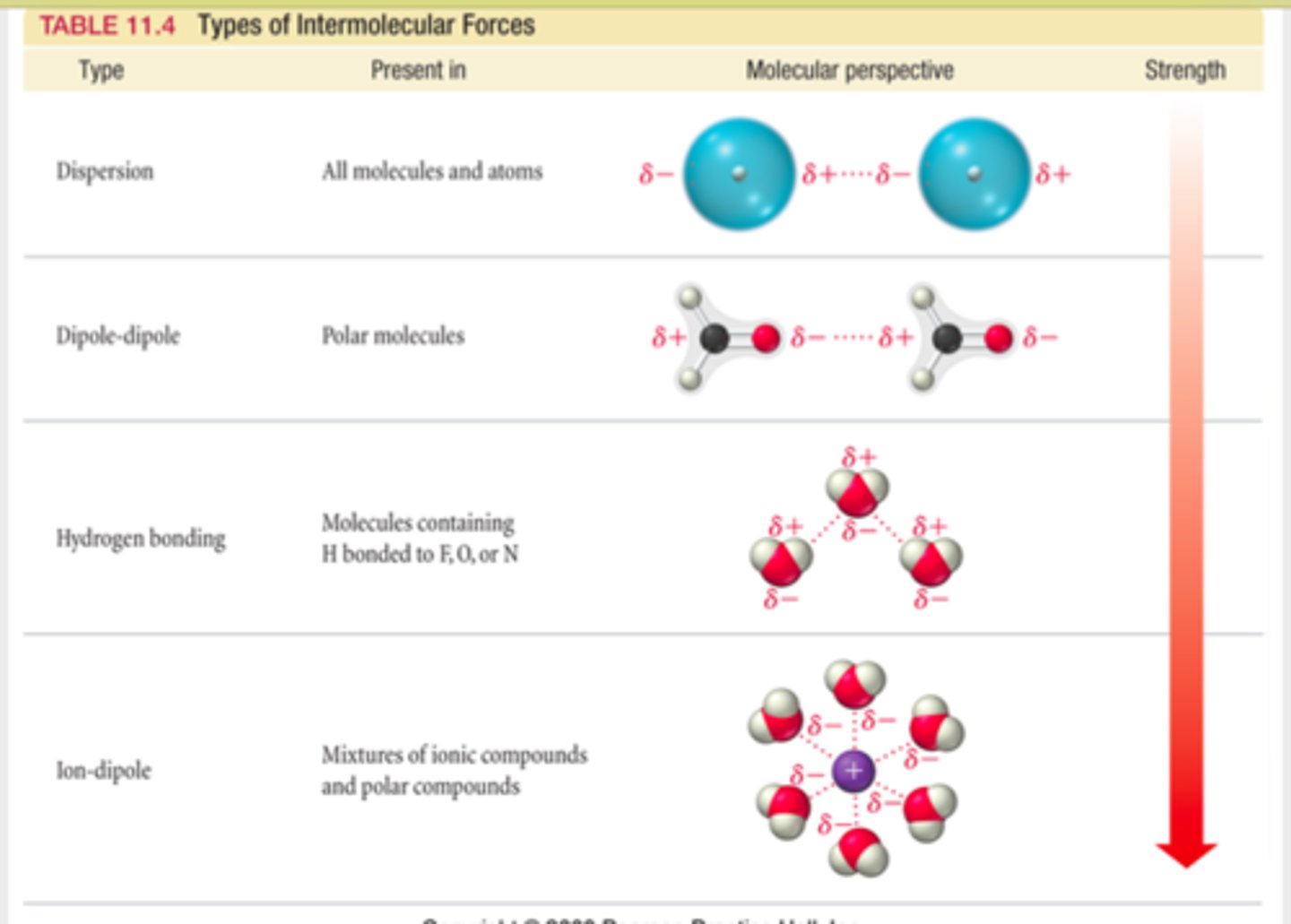
Types of intermolecular forces:
Dipole-dipole
Ion-dipole
Dipole-induced Dipole

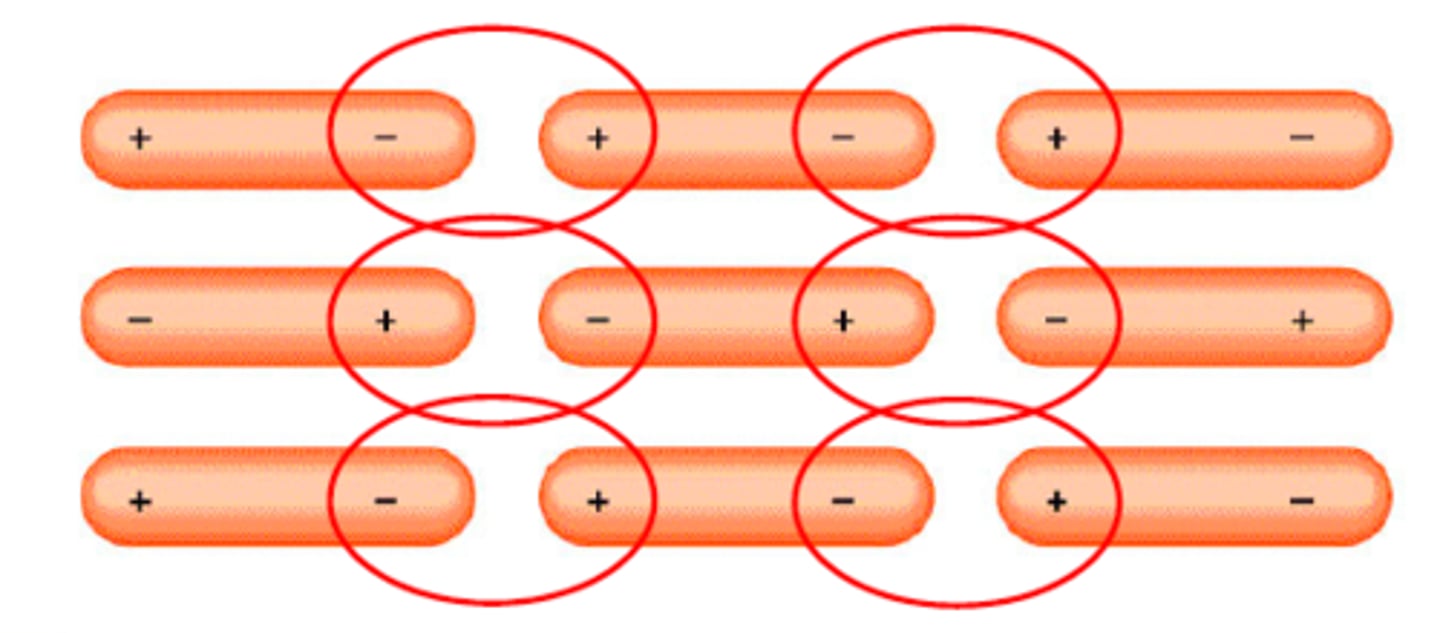
Dipole-dipole forces
forces between polar molecules (partially charged)- opposite poles match up by electrostatic attraction
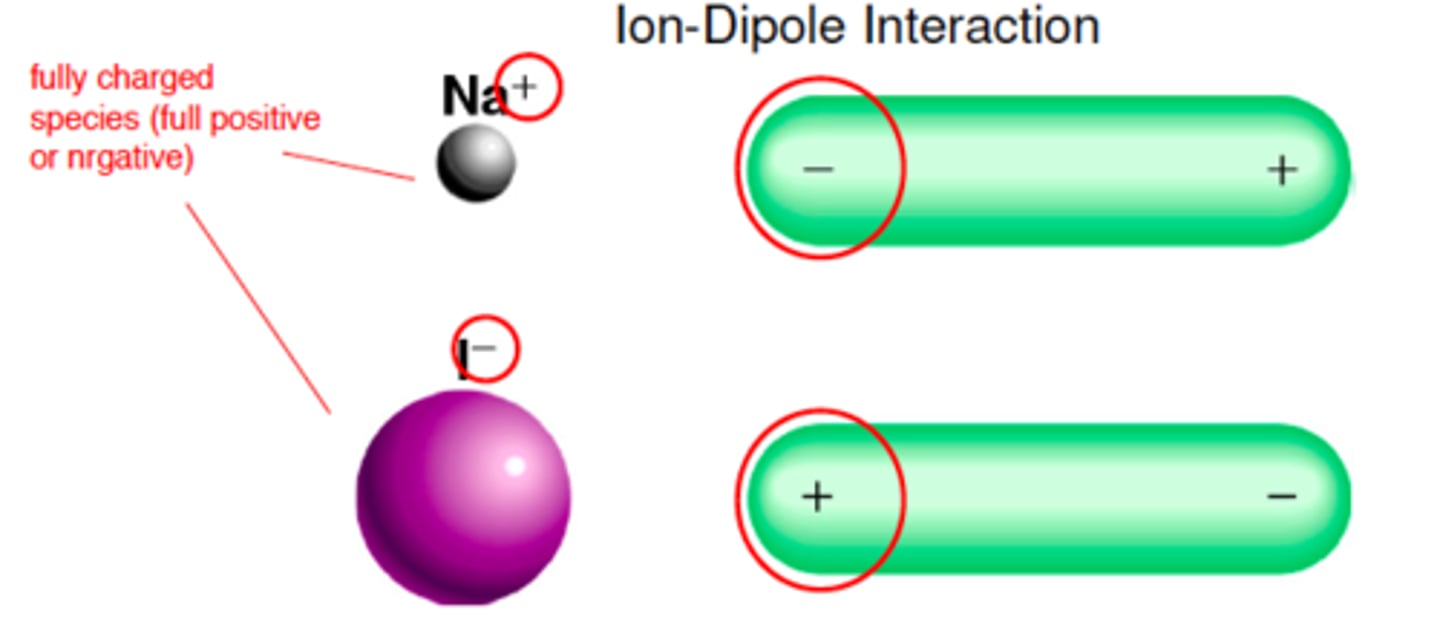
Ion-dipole forces
forces between a fully charged species (ion) and a dipole by electrostatic attraction.
Increase in strength with increase in charge or decrease in size of ion
(how ions are dissolved in water- attract to the H2O and pulled away by electrostatic charge)
Dipole-induced-dipole forces
forces arising from temporary dipoles induced in atoms by ions with a permanent dipole (either ion or dipole).
Temporary dipoles created when non-polar molecule interacts with polar- temporarily shifts the electrostatic charge to attract the polar molecule, then returns when removed

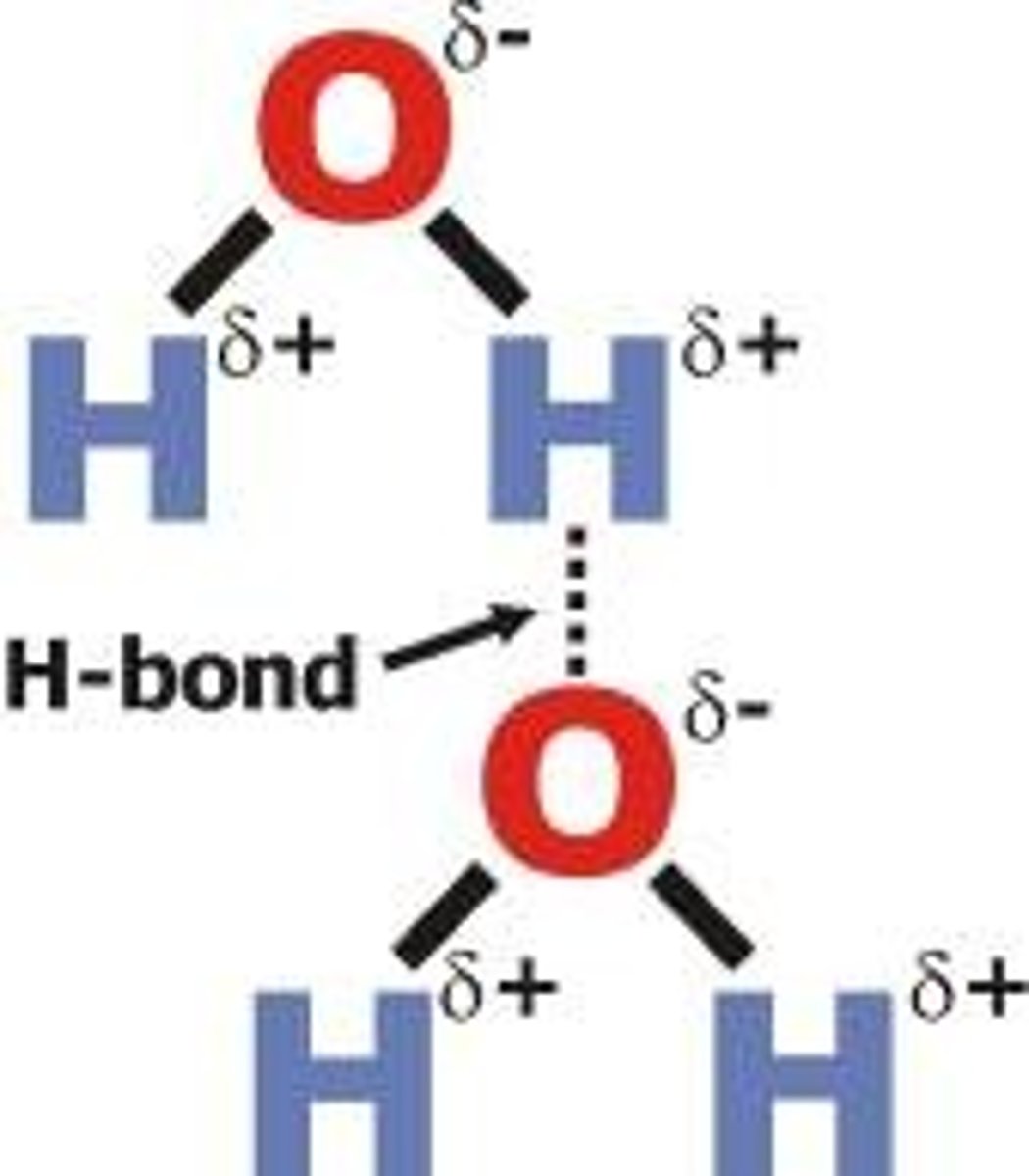
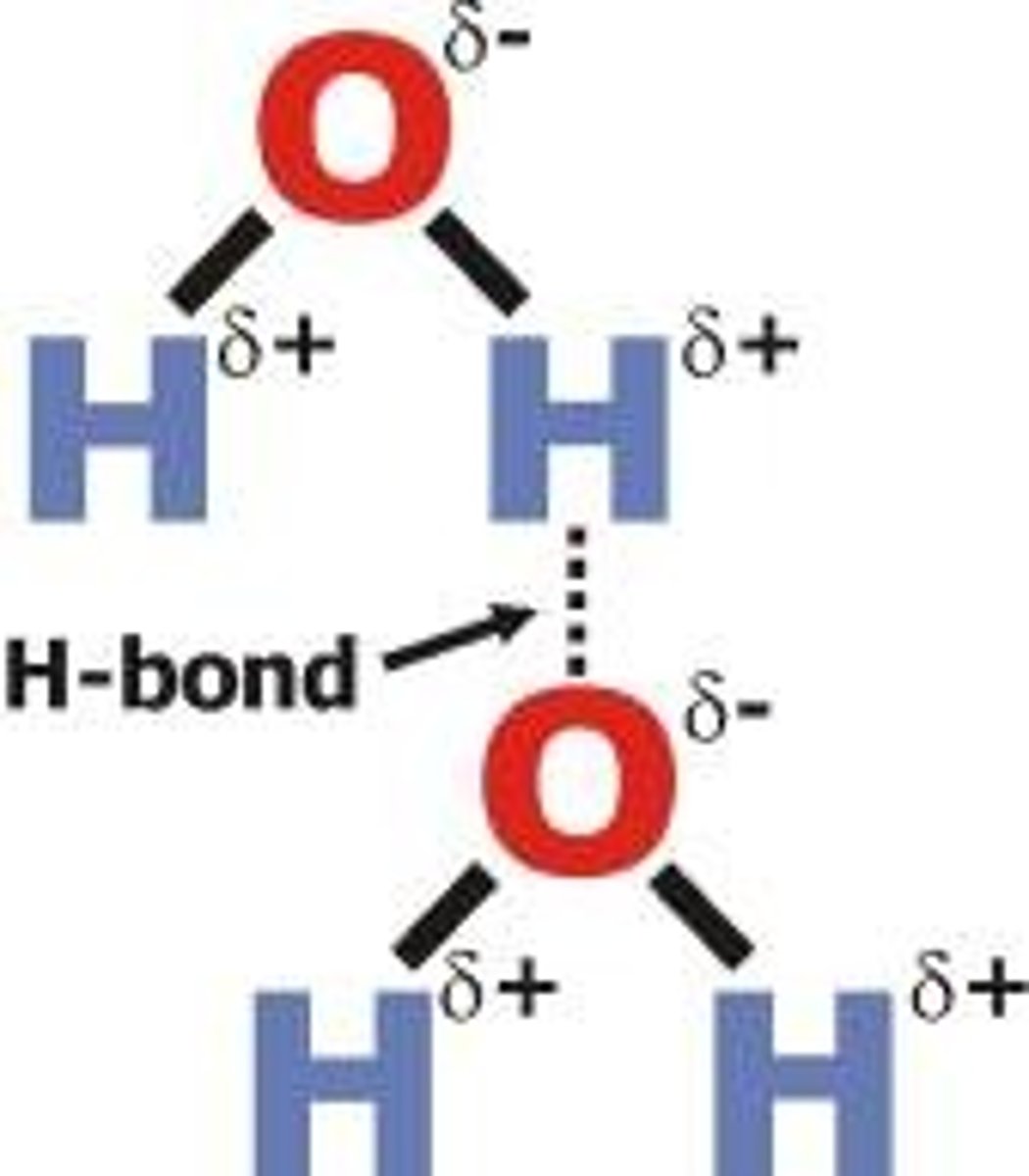
Hydrogen bonds
A special dipole-dipole interaction between a Hydrogen atom and either N, O, or F atom (smallest and most electronegative on PT)
- the lone pair on either N, O, F (in a molecule) is what causes the bond- strongest interaction with H
- reason why solid form of water floats on liquid form- H bonds- once frozen becomes less dense than liquid
surface tension
Surface tension is the amount of energy required to stretch the surface area of a liquid by unit area
- strong intermolecular force = strong surface tension

viscosity
Measure of fluids resistance to flow
- strong intermolecular force = high viscosity

solubility
- Polar compounds dissolve other polar compounds
- Non-polar compounds dissolve non-polar
- Polar compounds insoluble in non-polar

Intermolecular Force
Force between molecules
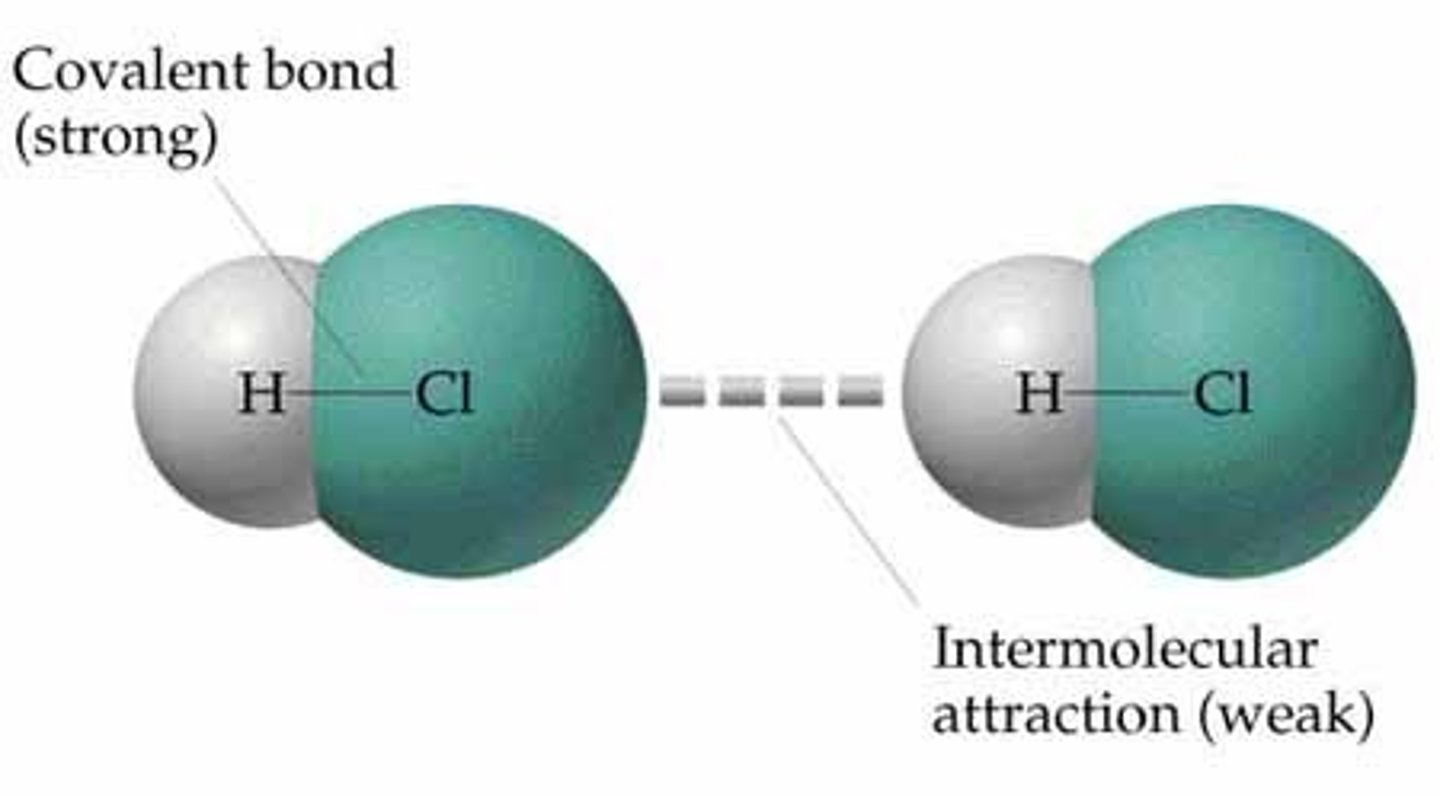
Intramolecular Force
Force within a molecule
Covalent Bond
Force of attraction within a molecule created by the sharing of electrons
Ionic Bond
Force of attraction created by the transfer of electrons between atoms
Polar Molecule
A molecule in which the covalent bonds are asymmetrically arranged
Nonpolar Molecule
A molecule in which the covalent bonds are symmetrically arranged
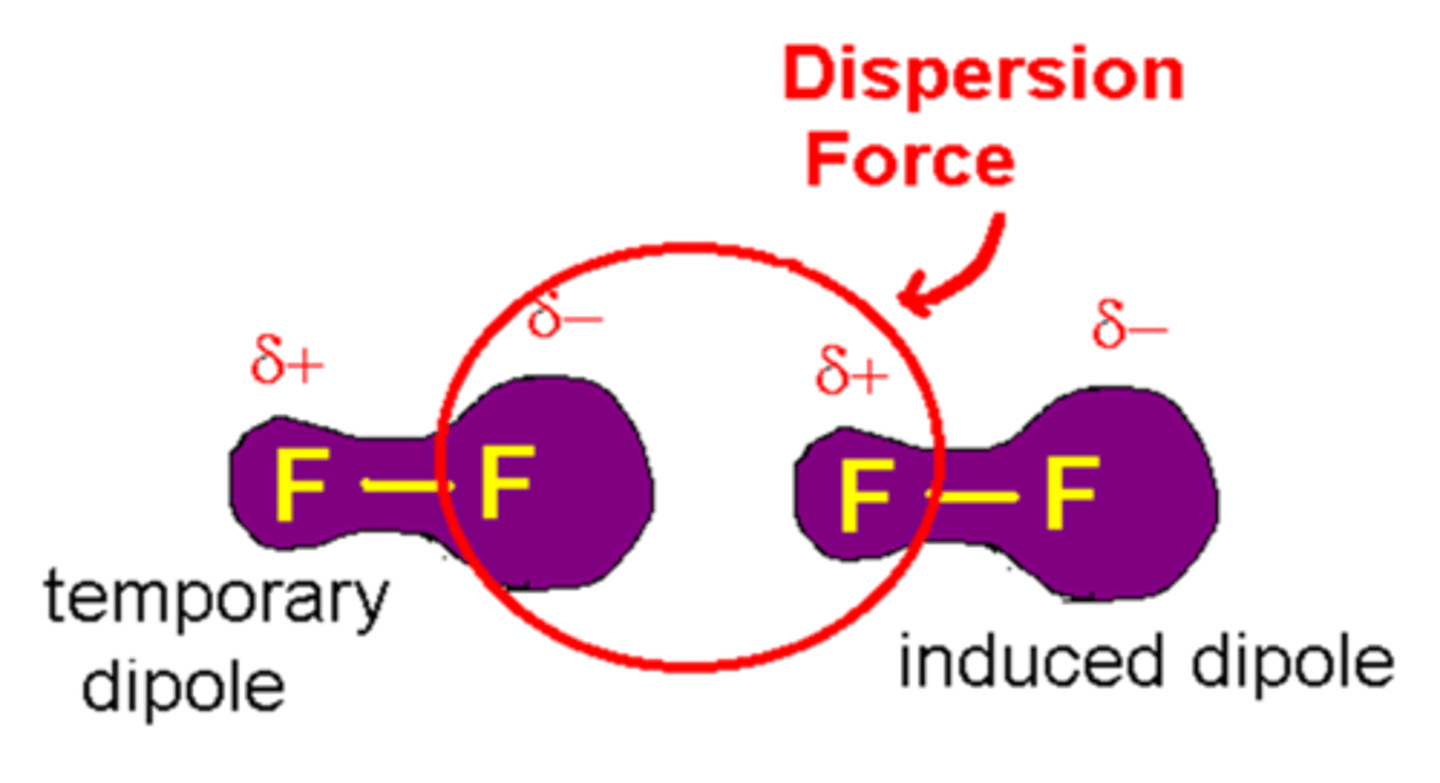
London Dispersion Force
Intermolecular force between nonpolar molecules
Heavier molecule = stronger force
Longer Chain molecule = more polarizable
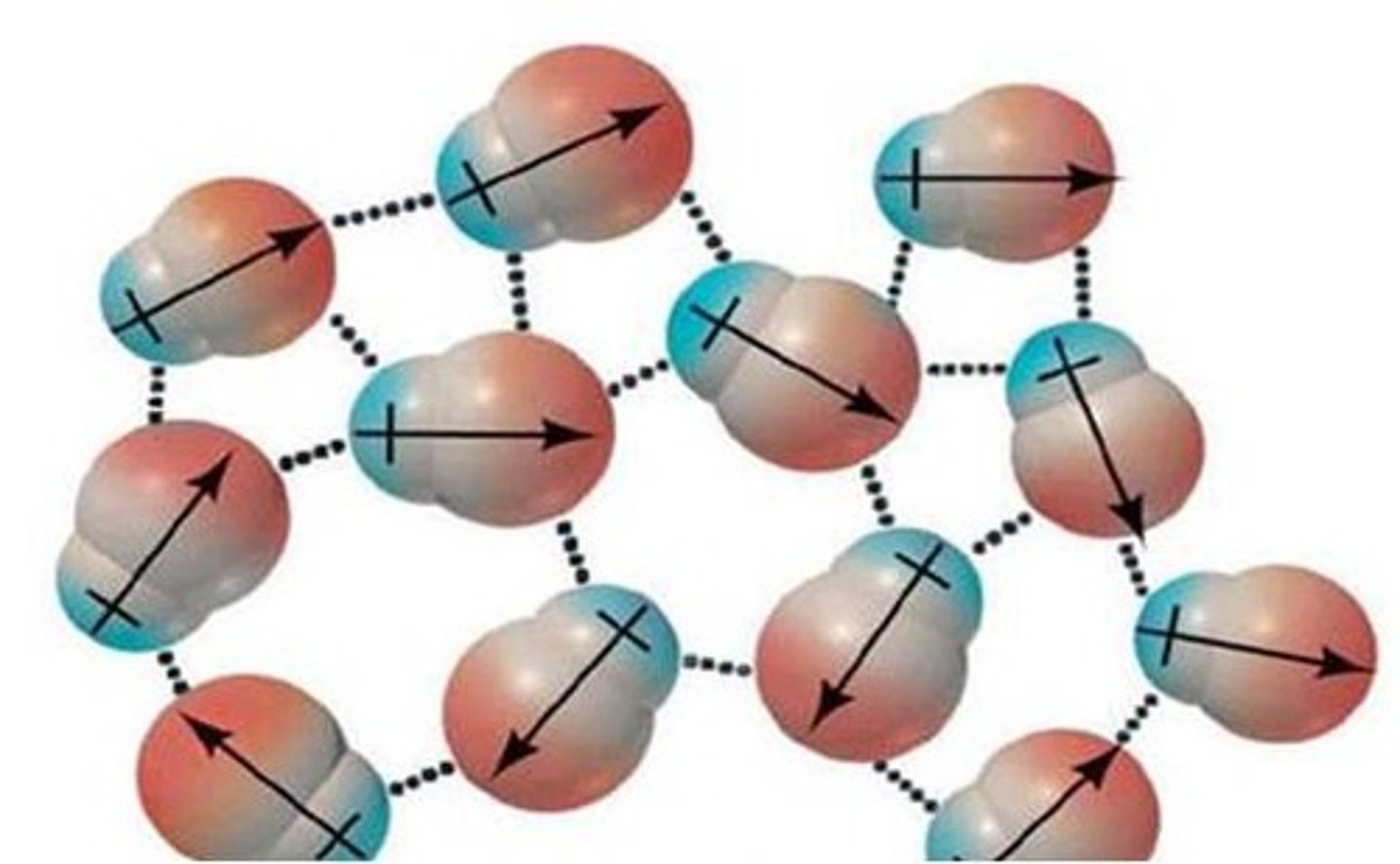
Dipole-Dipole Force
Intermolecular force between polar molecules
Heavier Molecules = More Polarizable=Stronger Forces
Hydrogen Bond
Intermolecular force between molecules containing hydrogen bonded to N, O, or F
More H-O, H-N, H-F connections= Stronger Force
Boiling point
Temperature at which the vapor pressure is equal to the atmospheric pressure

delocalized electrons
electrons that are free to move
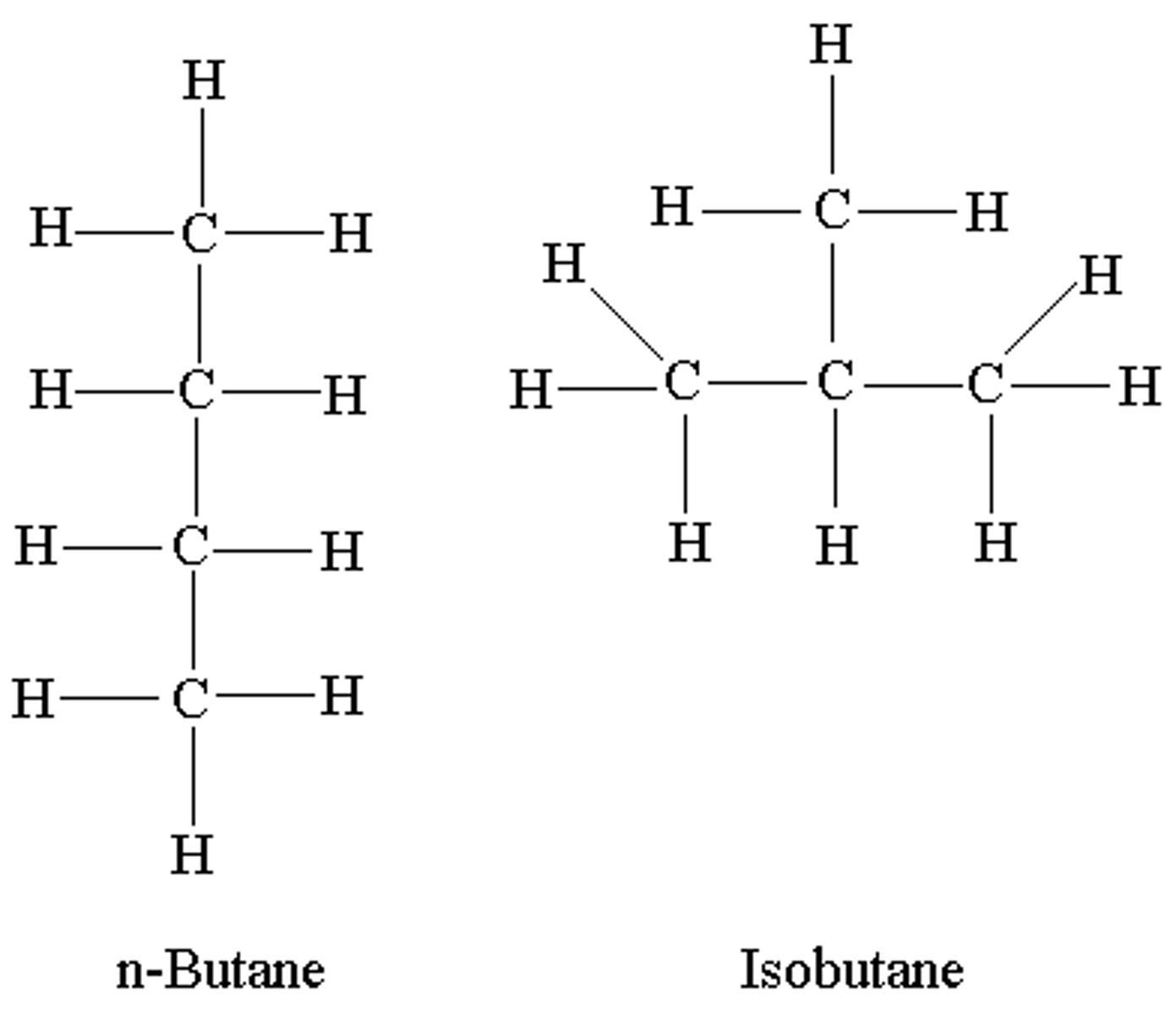
Isomers
Compounds with the same formula (mass)but different structures.
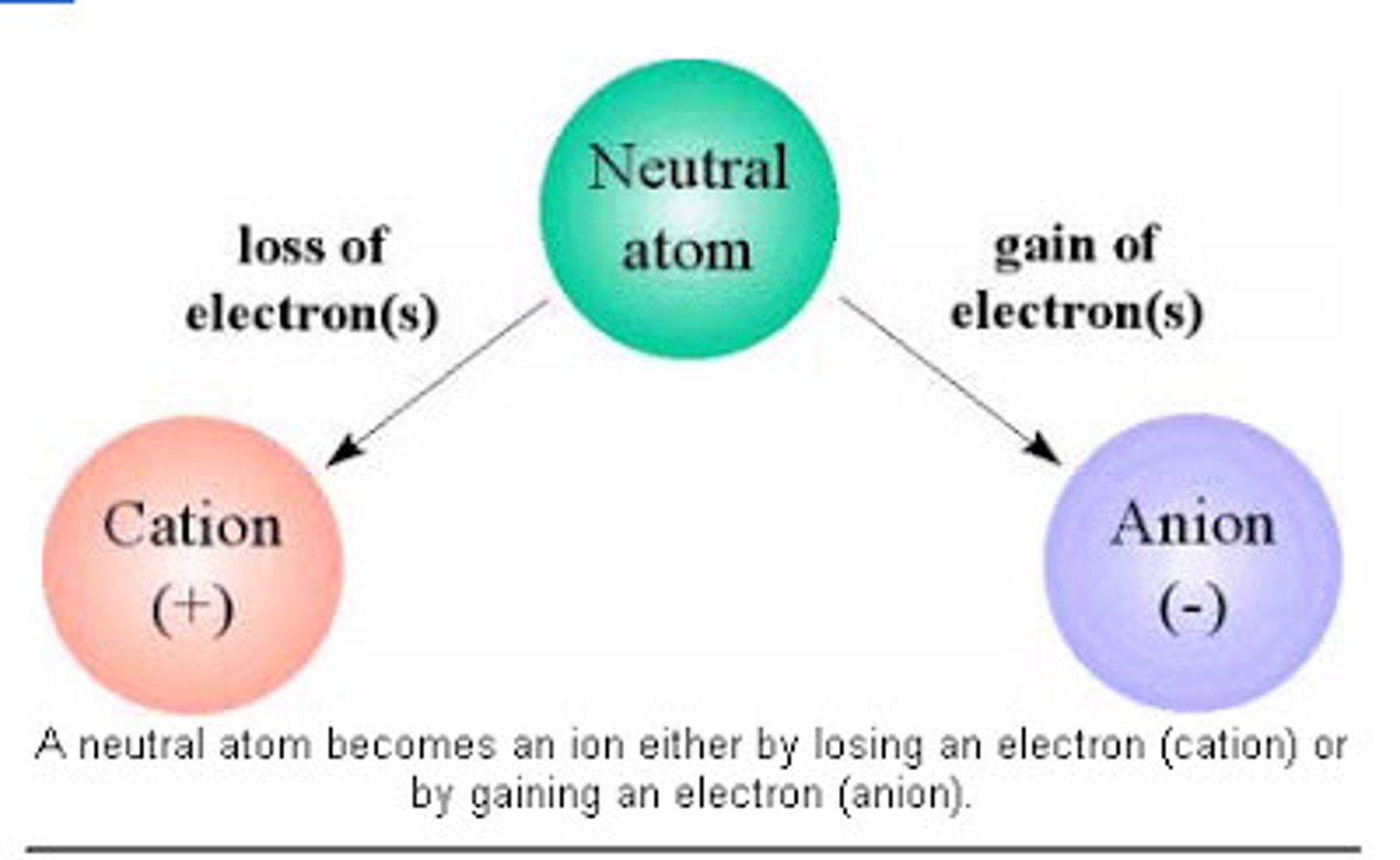
Cation
A positively charged ion
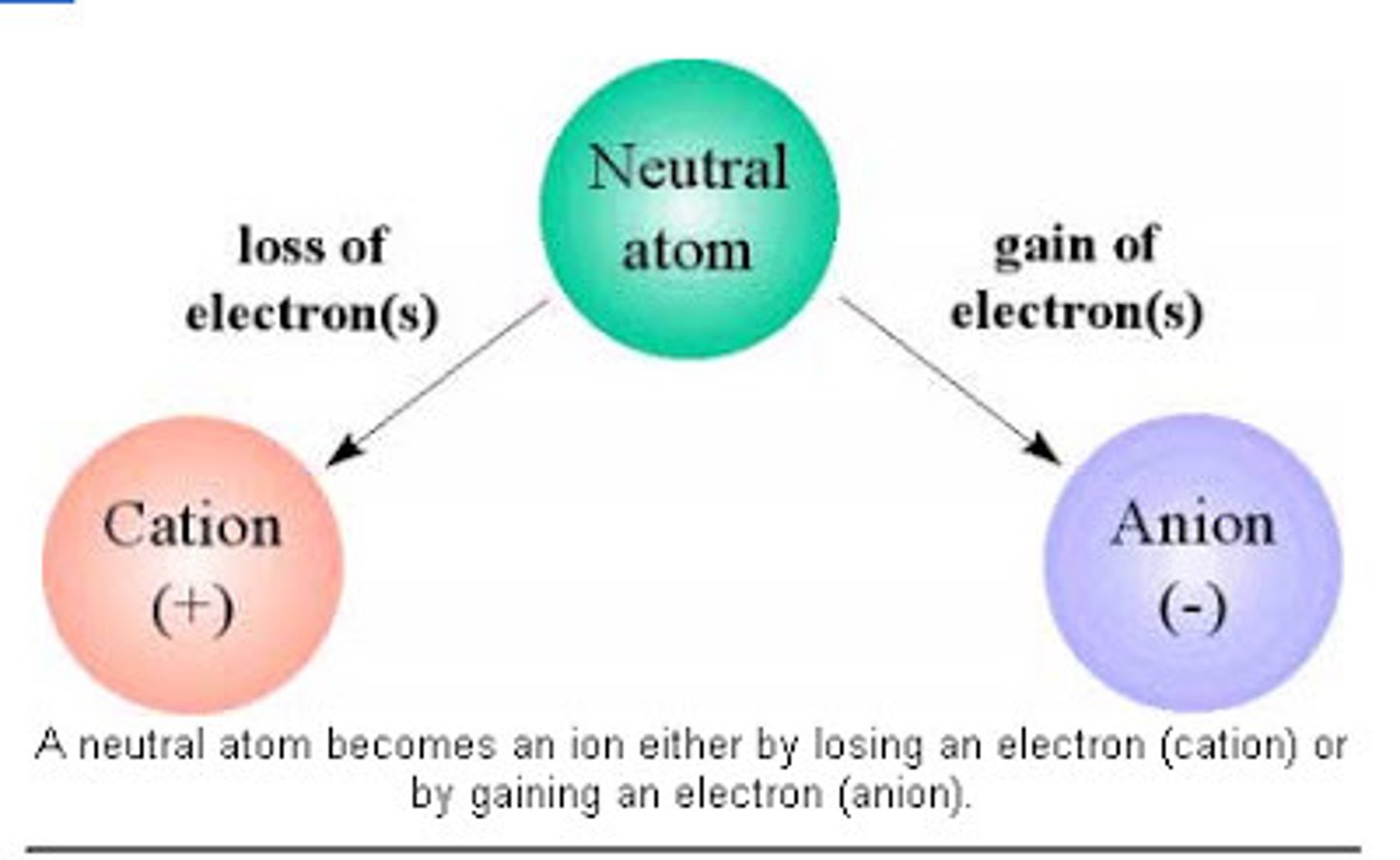
Anion
A negatively charged ion
ion-dipole interactions
occurs between ion (from an ionic compound) and a molecule from a polar covalent substance.
Can a London Force form between HI and HBr?
No, because they form dipole-dipole forces.
Forces between atoms in a compound are ______________ ______.
intramolecular forces
Forces that act between molecules are ______________ ______.
intermolecular forces
_____ _________ have very strong bonds (attractive forces) so they have high melting points and boiling points.
ionic compounds
________ _________ have lower melting and boiling points because covalent bonds are not as strong as ionic bonds.
covalent compounds
Generally, intermolecular forces are much ______ than intramolecular forces.
weaker
We can use _________________ to predict what kind of bond will form.
electronegativity
_____ _________ are like a battery, they have a positive and a negative end (like two poles of a planet or a magnet); the ends are opposite of each other.
polar molecules
A ______-______ _____ happens between the partial charges of polar molecules.
dipole-dipole force
The ____ polar the molecules are, the stronger the dipole-dipole forces between them, and thus, the higher the boiling point.
more
________ _____ involve the attraction between polar molecules that contain hydrogen and molecules that have N-H, O-H, or F-H bonds.
hydrogen bonds
________ _____ are very strong because hydrogen atoms are small and very polar (a large difference in electronegativity between atoms).
hydrogen bonds
Water has unique properties because of all the strong ________ _____.
hydrogen bonds
______ __________ ______ are nonpolar molecules, like F₂, which can become momentarily polar which results in a force of attraction between the molecules.
London dispersion forces
In ______ __________ ______, electrons are moving around in atoms, so there may be times when the electron distribution is not symmetrical).
London dispersion forces
___-______ ______ are the interaction or attraction between an ion and a polar molecule.
ion-dipole forces
An example of ___-______ _____ includes any ionic compound that dissociates into ions when dissolved in water (like NaCl in water or AgNO₃ in water).
ion-dipole forces
In _____, because the particles are so far apart, the attractive forces between them do not have a great effect.
gases
_____ are fluids and can flow easily because the particles move freely.
gases
In ______, particles can only vibrate in place and do not break away from their fixed positions.
solids
In ______, the particles are held very close together by attractive forces.
solids