Topic #1 Basics of Chemistry & Matter
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Elements
Fundamental building blocks of matter
Matter
Has mass & takes up space
Can be understood in terms of arrangements & rearrangements of atoms
Atoms retain their identity in chemical reactions
Except when a substance is radioactive (unstable nucleus)
Elements
Can be diatomic or monoatomic
Compounds
Can be binary or ternary
Substance
A form of matter that has a definite composition and distinct properties
Composed of atoms
Compounds form in definite proportion
ex. water
Chemistry
The study of changes in matter and the energy needed to make these changes.
States of Matter
Changes of state involving altering IMFs not altering actual chemical bonds.
Liquid —> Gas
Boiling, vaporizing, evaporating
Gas —> Liquid
Condensing, liquefying
Entropy
A measure of disorder or randomness in a system, or how spread out energy is.
Solid have the least entropy: Particles are in a tightly packed, highly ordered, fixed arrangement.
Gas has the most entropy: Particles are far apart, highly disordered, and have the most freedom to move.
Mixtures
No energy changes
Variable composition
Components retain their characteristic properties
Mixtures of different compositions may have widely different properties
Can be physically separated
Mixture → Pure substance
May be separated into pure substances by physical methods.
Pure substances
Fixed composition
Cannot be separated into simpler substances by physical methods
Can only be changed in identity and properties by chemical methods
Properties do not vary
Mixtures can be?
Can be evenly distributed or not uniform
Homogeneous mixture (evenly distributed)
Have same composition throughout
Components are visibly indistinguishable
Solutions ~ gaseous & aqueous! Solute in solvent
Heterogeneous mixture
Do not have the same composition throughout
Components are visibly distinguishable
Filter insoluble residue
Pure substance can be?
Decomposed or can’t be decomposed
Pure substances that can be decomposed are compounds
Can be decomposed into simpler substances by chemical changes. Always in a definite ratio.
Pure substances that can’t be decomposed are elements
Cannot be decomposed into simpler substances by chemical changes.
Diatomic Elements
Br, I, N, Cl, H, O, F
Allotropes
Different forms of the same element. Different structure & properties.
ex. diamond and graphite
Evaporation (A method to physically separate a mixture)
Separating a mixture (solution) of a soluble solute and a solvent (salt in water).
Heating the solution until the solvent evaporates leaving behind the solid residue.
Fractional Distillation (A method to physically separate a mixture)
Used to separate a solution of 2 or more miscible (soluble) liquids by varying boiling points.
Chromatography
Based on solubility.
The solvent and paper both have an attraction for the components in a mixture. If a material is placed on one spot on the paper and is soluble in the liquid solvent, the material will be dissolved when the solvent moves over it.
When we get to solutions ~ Like dissolves like.
Compounds
A pure substance composed of atoms of two or more elements chemically combined in fixed proportion.
Binary compound
2 types of elements
Ternary compound
3 types of elements. May have a polyatomic ion.
Compounds can only be separated into their pure compounds (elements) by…
Chemical changes.
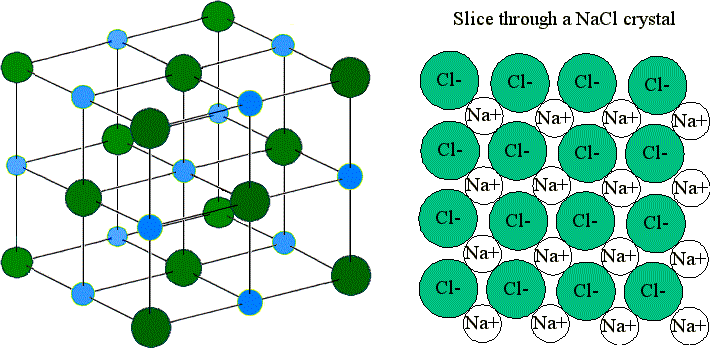
Ionic compound
Form one crystalline lattice structure
Metal (cation) + Nonmetal (anion)
Transfer of electrons
Molecular compounds
Soft substances, poor conductors
Molecules held together by covalent bonds and intermolecular forces.
London Dispersion (VDW) ~ nonpolar
Dipole-Dipole ~ polar
H-Bonds ~ H with F, O, N
Extensive Property
Depends upon how much matter is being considered.
ex. mass, length, volume
Intensive Property
Does not depend on how much matter is being considered.
ex. density, temperature, color
Intermolecular forces
An attraction between two molecules.
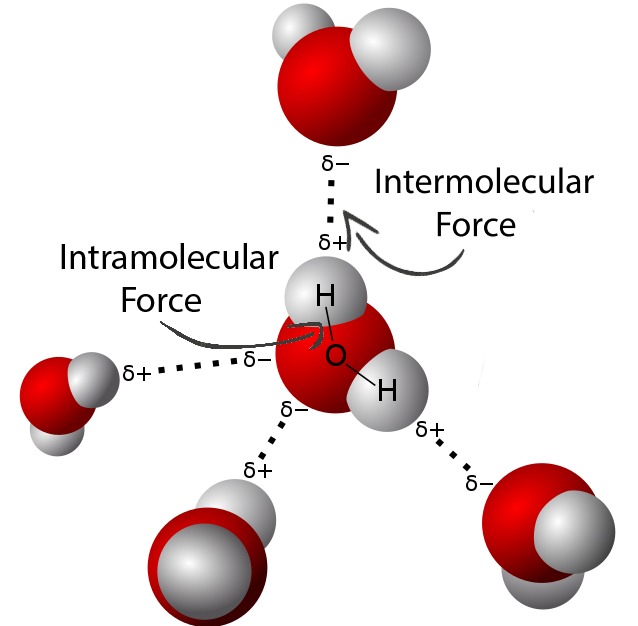
Intramolecular force
A bond within a molecule that holds the atoms together.
London Dispersion Forces
The weakest attractive forces between molecules or atoms are caused by shifts in the distribution of electrons, which creates temporary uneven charges (temporary dipoles).
Stronger in larger atoms and molecules
Present in all substances
Dipole-Dipole Forces
Attraction between molecules with permanent partial charges (polar molecules).
Hydrogen Bonding
A stronger type of dipole-dipole force which occurs in molecules with bonds of H with F, O, and N.
Ion-Dipole
Attractions that occur between a charged ion and a polar molecule.
Mole
A unit used by chemists to express the amount of a compound or element.
Avogadro’s number
6.02 x10^(23), the number of particles (molecules, ions, atoms) in a mole of any substance.
Molecular implies what?
Covalent bonding
Empirical Formula
The most reduced form of a chemical formula. The ratio necessary for all ionic substances.
The Law of Constant Composition
States that any sample of a pure compound always consists of the same elements combined in the same proportions by mass.
Synthesis
Atoms of elements combine to form a compound.
ex. 4Fe + 3O2 —> 2Fe2O3
Decomposition
Compound is broken down into its component elements or simpler compounds.
ex. 2H2O —> 2H2 + O2
Single Replacement
A single element reacts with a compound to take the place of an element of similar character. This results in the formation of a new compound and a new free element.
ex. Cu + 2 AgNO3 —> Cu(NO3)2 + Ag
Double Replacement
Two compounds react to produce two new compounds (elements switch places).
ex. AgCl + KNO3 —> AgNO3 + KCl
Combustion
CH and/or O + O2 —> CO2 (g) + H2O (g)
The Law of Conservation of Matter
Matter can be neither created nor destroyed.
Coulombs Law
Quantifies the amount of force between two objects.
Magnitude of the electrostatic force of attraction or repulsion between two points is directly proportional to the product of the magnitude of the charges.
Attraction is inversely proportional to the distance between them.
Stoichiometry
The study of quantities of materials consumed and produced in balanced chemical reactions.
Math done to change a substance to another substance
All carbonates break down to?
The metal oxide and carbon dioxide gas.
Na2CO3 —> Na2O + CO2
All chlorates break down to?
The metal chloride and oxygen gas.
Ba(ClO3)2 —> BaCl2 + 3 O2
Metal hydroxides break down to?
Metal Oxide + Water
Cu(OH)2 —> CuO + H2O
Oxy acids break down to?
Non-metal oxide (with the nonmetal having the same valence) + Water
2H3PO4 —> P2O5 + 3 H2O
Any time H2CO3 is formed it results in…
—> CO2 + H2O
Metal oxide + Carbon dioxide forms what?
ex. K2O + CO2 —> ?
Metal carbonate
K2O + CO2 —> K2CO3
Metal chloride + Oxygen forms what?
ex. BeCl2 + 3 O2 —> ?
Metal chlorate
BeCl2 + 3 O2 —> Be(ClO3)2
Precision
Reproducibility; agreement of several measurements
Accuracy
Correctness; agreement of a measurement with the true value
Random/indeterminate error
Equal probability of a measurement being high or low
Systematic/determinate error
Occurs in the same direction/type each time