Chapter 2 - Polar Covalent Bonds, Acids and Bases
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
Ionic bonds
Ions held together by electrostatic attractions between unlike charges
Bond in sodium chloride
Sodium transfers an electron to chlorine to give Na+ and Cl-
Nonpolar Covalent bonds
Two electrons are shared equally by the two bonding atoms
Carbon-carbon bond in ethane
Symmetrical electron distribution
Polar covalent bonds
A covalent bond in which the electron distribution between atoms is unsymmetrical.
This is due to electronegativity (EN)
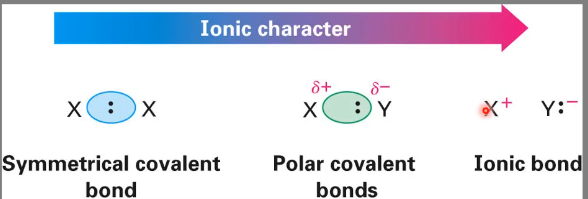
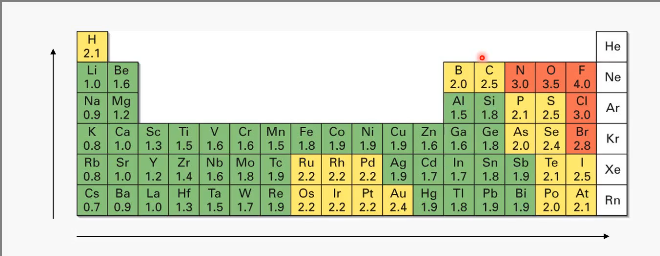
Electronegativity trends
Higher number of electronegativity = higher ability of an atom to attract shared electrons in a covalent bond.
Increases across the periodic table from left to right and from bottom to top.
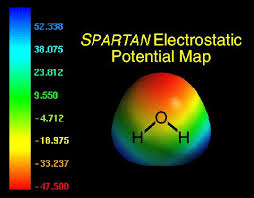
What is an electrostatic potential map?
Shows calculated charge distribution
Red: Electron rich. Blue: Electron poor.
Arrow is used to indicate direction of bond polarity
Electrons are displaced in the direction of the arrow.
So arrow goes from electron poor area to electron high area
Symmetrical structures
Symmetrical structures of molecules cause the individual bond polarities and lone-pair contributions to exactly cancel.
Formal charge
If the number of bonds is different than the usual amount, a formal charge can be assigned.
+ when the atom has one electron less than usual
- when the atom has one electron more than usual
Formula formal charge
Formal charge = Number of valence electrons in free atom - (number of bonding electrons/2) - number of nonbonding electrons
Rules for resonance
Individual resonance forms are imaginary, not real (real structure is made up of several elements)
Resonance forms differ only in the placement of their pi or nonbonding electrons.
Different resonance forms of a substrate do not have to be equivalent.
Resonance forms obey normal rules of valency (follow the octet rule/valid Lewis structure)
The resonance hybrid is more stable than any individual resonance form.
Drawing resonance
In general any three-atom grouping with a p orbital on each atom has two resonance forms
The actual structure of the resonance hybrid is closer to the more stable form.
Bronsted-Lowry acid/base
A Bronsted Lowry acid: substance that donates a hydrogen ion (H+) to a base.
A Bronsted Lowry base: a substance that accepts a hydrogen ion from an acid
Lewis acid/base
Lewis acid: A substance with a vacant low energy orbital that can accept an electron pair from a base (All electrophiles are Lewis acids)
Lewis base: A substance that donates an electron lone pair to an acid. (All nucleophiles are Lewis bases)
Lewis acids
To accept an electron pair a Lewis acid must have a vacant, low-energy orbital
H+ is a Lewis acid, can accept a pair of electrons with an empty 1s orbital.
Various metal cations, such as Mg2+, are Lewis acids because they accept a pair of electrons when they form a bond to a base.
An atom, ion, or molecule with an incomplete octet of electrons can act as an Lewis acid.
Molecules where the central atom can have more than 8 valence shell electrons (e.g. transition metals) can be electron acceptors, and thus are classified as Lewis acids.
Lewis base
a compound with a pair of nonbonding electrons that it can use in bonding to a Lewis acid
Definition of Lewis base similar to Bronsted-Lowry definition
H2O acts as a Lewis base, has two nonbonding electrons on oxygen.
Noncovalent interactions
Also called intermolecular forces or van der Waals forces
Dipole-dipole forces (between polar molecules)
Dispersion forces (between all neighboring molecules)
Hydrogen bonds
Two bonding (sp) bond angle
180, linear
Three bonding (sp2)
120, trigonal planar
Four electron domains (sp3)
107, tetrahedral