12.1 Notes
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
What is Sustainable Chemistry. Examples.
Sustainable chemistry aims to balance the need for economic growth, the desire to protect the natural environment, and the responsible of looking after people’s health and wellbeing.
Approaches to sustainable chemistry include, using renewables, locally sourced materials and designing process to minimize waste and energy consumption.
For example, right now, Australians are trying to balance mining for non-renewables (coal, oil and natural, gas), which are local sourced materials and help the economy with desire to produce to more renewables, which might not be locally sourced but benefit the environment in the long term.
Environmental Chemistry
The study of chemical processes that occur in the natural environment and how these processes are impacted by human actives.
An example of environmental chemistry is studying how certain sea creatures (like shellfish) use calcification to survive and how calcification is negatively impacted by the excess carbon dioxide human have release into the atmosphere.
Green Chemistry. Examples
Sustainable and environmentally friendly chemical practices. With the ultimate goal being to design more resource-efficient, environmentally friendly products.
The approach to green chemistry can be summarized by the “12 principles of green energy”.
Prevent waste
Means to design chemical processes that prevent waste rather than clean up waste afterwards.
For example, recycling reactants, like is done in the Haber process.
Maximize the “Atom Economy”
Design chemical process that are highly efficient
Meaning that the final product contains the maximum proportion of starting atoms, minimizing the wasted atoms.
Designing less hazardous chemical synthesis process
Designing safer methods that use/generate less harmful materials.
For example, the process of extracting gold in Australia.
Design safer products and chemicals
Design product that are effective yet are not harmful to humans or the environment.
For example, using carbon dioxide as a detergent to clean grease instead of perchloroethylene (PERC)
or using ammonia or carbon dioxide as refrigerant instead of chlorofluorocarbons (CFCs)
Use safer solvents and reaction condistions
Avoid using toxic solvents to dissolve reactants. Like for example avoiding the use of cyanide in gold extraction and using glycine instead.
Increase energy efficiency
Using minimum energy requirements only.
Conducting synthesis at ambient temperature and pressure
Catalysts might be useful, depending on the price and abundance of the catalyst.
Use renewable materials
Using renewable resources,
like plant-based materials
Instead of non-renewables
like fossil fuels
For environmental and cost reasons
Avoid “chemical derviatives”
Avoid using multi-step equations and making chemicals from other chemicals when possible
when it cost-effective and safe.
As this reduces the extra reagents needed and the potential waste produced.
Use catalysts not excess reactants to increase rate of reaction
The is used to reduced waste, as catalysts are reusable.
Design chemicals and products that are biodegradable
They break down into harmless chemicals after use and don’t accumulate in the environment
This reduces the environmental impact
For example, avoiding using cyanide in gold extraction.
Analyse in real time to prevent pollution
Continuous monitoring and control during the process to minimize or climate the formation of by-products which can be harmful to the environment or humans.
Minimize potential for accidents
Design chemicals, their states (solid, liquid or gas) and how they are stored, to minimize potential accidents.
like explosions, fires or releases into the environment.
For example, highly reactive (with water) metals are kept in oil to prevent them from catching fire and exploding.
Or storing nitrogen is gaseous, not liquid, form.
Green synthesis
Synthesis
Designing efficient methods for converting readily available starting materials into desired products.
Green
means environmentally friendly and sustainable
Real world example of chemical synthesis - the production of insulin
Insulin is a drug used to treat diabetes
In the early 1920s, the only source of insulin was living organisms - pigs and cattle etc.
the insulin was extracted purified an injected to people.
This process was long and raised economic and environmental concerns.
In the early 1950s, however, the amino acid structure of insulin was characterized.
this made chemical synthesis possible.
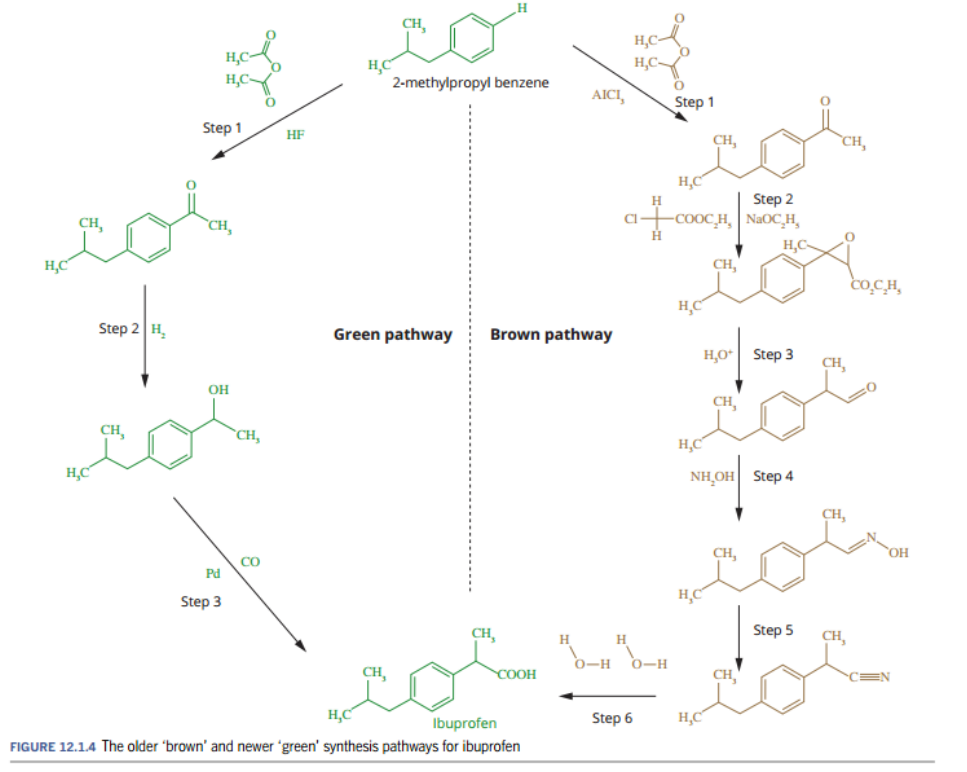
Example of green synthesis - Ibuprofen
Relate to the Principles of Green Chemistry
Nurofen is a popular over the counter pain killer. It is the active ingredient is Ibuprofen.
In the 1950s
Ibuprofen was synthesized using a “brown” synthesis pathway
Involved six steps
In which only about 40% the atoms in the reactants ended up in the final product, the rest were waste.
This not cost-effective and environmentally friendly.
And doesn’t align with certain principles of green chemistry like 1 and 2,
which are to prevent waste, and maximize the atom economy
In 1992,
a new “green” synthesis pathway was developed
Only involved three separate steps,
in which 77% of the atoms from the reactants resulted in final product.
This value can be increased to around 99% by the regeneration and reuse of some reactants.
This very cost-effect and environmentally friendly.
And does align with certain principle of green chemistry like 1 and 2
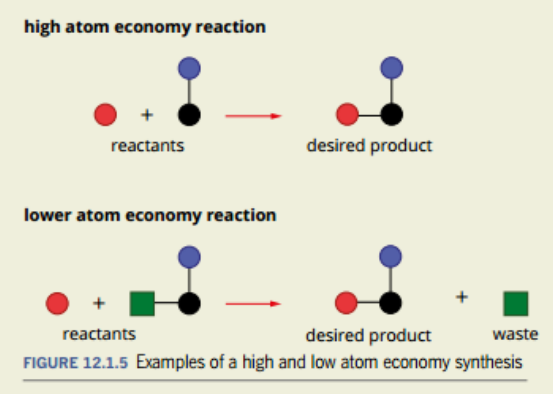
The Atom Economy
Is a way of accounting for the use of materials in the manufacturing process
Why is the Atom Economy Important and how does it adhere to the principle of green chemistry.
It is important because it calculates the number of atoms in the reactants that actually form into products.
It is also important to work out the amount of waste that you are producing and how efficient your process is.
It adheres to principle 2 of Green Chemistry, which is to maximize the atom economy
What is one way of calculating the atom economy
Atom Economy = M (Desired product) / M (All reactants) *100%
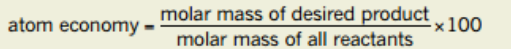
Another way of calculating atom economy
Atom Economy = m (desired product) / m (all reactants) *100%

Green chemicals PERC vs CO2: What is PERC?
Perchloroethylene (PERC) is perfectly non-polar dissolving hydrophobic (insoluble) compounds like oil and grease
It is also liquid at room temperature, making it good for dry cleaning.
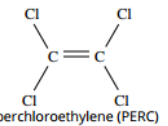
Disadvantages of PERC. Which principles of green chemistry does it violate.
PERC is carcinogenic (can cause cancer)
Is linked to conditions like Parkison’s disease.
This is in breach of green chemistry principle 4
which is to design safer chemicals and products.
Green chemicals PERC vs CO2: What is CO2? Why it is better?
CO2 - like PERC - is perfectly non-polar
meaning it too is good at dissolving fats and other hydrophobic (insoluble) things
making it good at dry cleaning
Not dangerous like PERC, adhering to the 4th principle of green chemistry which is to design safer chemical and products.
The disadvantages of CO2
At room temperature, CO2 is a gas and PERC is a liquid. Meaning PERC is more effective detergent.
And simply cooling CO2 to -80 degrees Celsius (when CO2 becomes a liquid) can damage fabric.
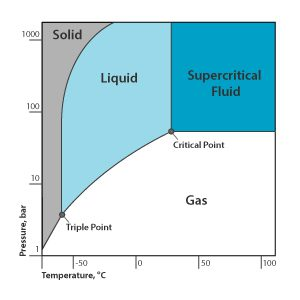
What to the Chemists chose to do then
They chose Supercritical CO2
Which at certain temperatures and pressure can behave like both a gas and a liquid.
And even though Supercritical CO2 can be difficult and expensive to maintain is still used as it is a safer alternative to PERC, which is why it is used
Antibacterial Handwashes
Triclosan was developed in the 1960s and was thought to be an effective antibacterial and antifungal agent
Side effects of Triclosan and why the US food and drug Admiration banned it. What principles of green chemistry did it violate.
US food and drug administration found no evidence that triclosan in soap no evidence that Triclosan in soap provided any benefit compared to washing hand with soap and water.
The potential side effects are not yet known.
Violates principle 4 of green chemistry, which is to design safer chemicals and products.
In 2016 a study found that plant wanted with triclosan contaminated water still had triclosan in them months later.
Violates principle 10, which is to products that are biodegradable and don’t accumilate in the environment
What is leaching
The process of extracting certain substances from a solid by dissolving them in liquid. An example of leaching is the extraction of gold using a cyanide solution.
Environmental impacts of mining for gold and the principle of green chemistry it breaks.
Cyanide is used. It is
Toxic to humans,
Stays in the environment for a long time
Is very expensive to dispose of properly.
This violates the green chemistry principles:
Number 1, prevent waste
Number 3, Design less hazardous chemical synthesis
Number 10, to design chemicals and products that are biodegradable and don’t accumulate in the environment
.
What did the Chemists at Curtin University do and how does it adhere to the principle of green chemistry.
Developed a gold extraction process that involved glycine (a type of amino acid): Glycine is
Cheaper than cyanide
Not toxic, unlike cyanide
Reuseable
Not dangerous of the environment, unlike cyanide
More abundant than cyanide
Can be used in in situ mining, reducing the need for huge dug out mines like the Super Pit.
This adheres to green chemistry principles 1,3, and 10 and also cuts production costs
What are trailings?
Trailings are material left over after valuable minerals.
They are normally stored in damns or large ponds
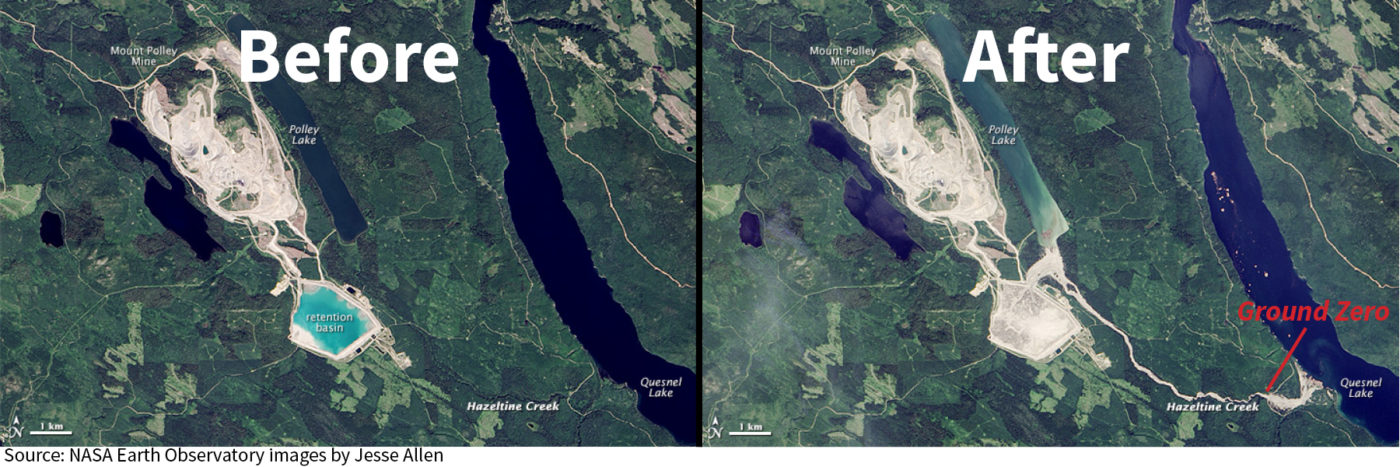
The 2014 Mount Polley Mine Disaster
4th of August 2014 in Canada
Breach in the trailings pond at the Mount Polley copper and gold mine
10 million m2 of waste was released
4.5 million m3 of trailing was released
The waste and trailings poured into the nearby Polley Lake and then the lake emptied to the Quesnel Lake.
Released
326 Tonnes of Nickle
400 Tonnes of Arsenic
177 Tonnes Lead
18400 Tonnes of Copper
Most of which ended up in the local environment
Example of Waste minimization and remediation: carbonation
“Red mud is a trailing product after the extraction of alumina (which is used to produce aluminum) for bauxite ore.
It is highly alkaline (pH >7) which is not good for the environment
Carbon dioxide is the product of combustion (and is also not environmentally friendly). It can be used to neutralize the “red mud”
This process is called Carbonization
Where is Carbonation Useful
Beneficial additive (Alkaloam) for Perth’s sandy soils
Increases phosphate retention by 70%
Can be used to treat eutrophication* in the Peel-Harvey Estuary, since there is no more reliance on phosphate fertilizers.
Another example of preventing waste: Turning Loster shells into a valuable manufacturing process and what green chemistry principles it adheres to.
What is so useful about Lobster shells
Chitin is a long chain polymer that can be found in Lobster (among other things like the cell walls of fungi).
It can be used in a variety of different industrial processes, like pharmaceuticals and manufacturing bioplastics.
How is Chitin extracted
Microwaves are used as a heat source
Lobster shells can be taken straight from the bins of kitchens
What green chemistry principles does this adhere to
Number 1, prevent waste
Number 3, Design a less hazardous chemical synthesis
Number 5, use safe solvents and reaction conditions