5.3 Wave - Particle Duality
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
De Broglie's Theory
Light exhibits both wave and particle behaviour instead of being restricted to just one.
He related wavelength to the momentum of a particle: λ = h/p
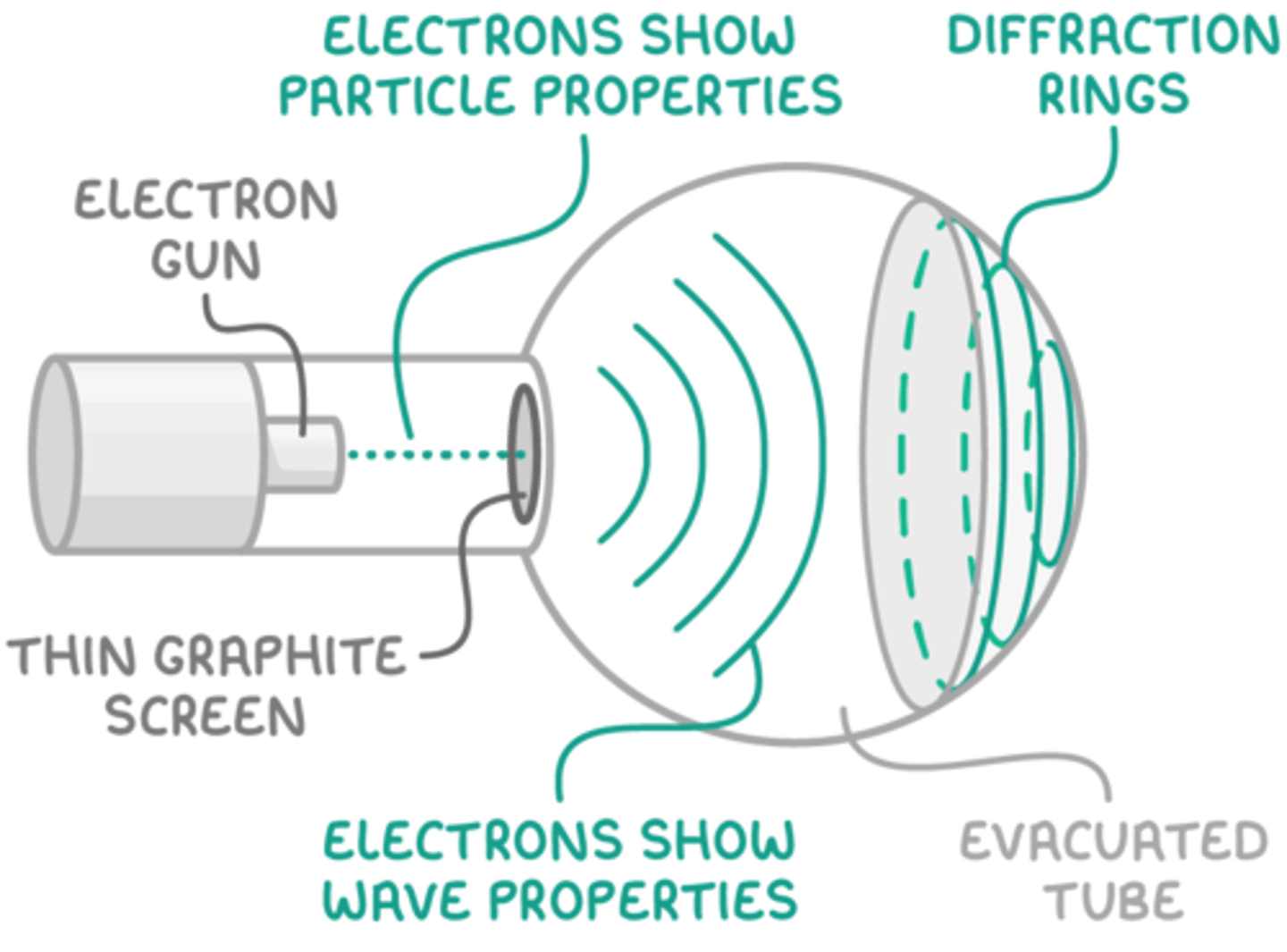
Electron Gun Experiment
An electron gun produces a focused beam of electrons by heating a filament and accelerating electrons through a high voltage.
The beam can be directed and used to study electron behavior, such as in electron diffraction or cathode ray tubes.
It demonstrates electron properties like charge, mass, and wave-like behavior when combined with diffraction experiments.
Electron Diffraction
We can fire electrons using an electron gun at polycrystalline graphite (many thin layers of graphene). The distance between the carbon atoms is similar to the wavelength of electrons which causes the electrons to diffract.
How does the electron gun experiment demonstrate wave particle duality?
They initially behave as a particle as they are accelerated by the high potential difference.
They then act as a wave when they diffract.
They then act again as particles when they collide with the screen.
Observable Wave Properties of Electrons
Faster electrons diffract less than slower electrons. Electron diffraction patterns are similar to x-ray diffraction patterns.
This evidence supports electron waves which have:
Shorter wavelengths for faster moving electrons
Longer wavelengths for shorter electrons
Electron Gun - Evidence for Particle Theory
The electron gun fires a beam of accelerated electrons that travel in straight lines and hit a screen at specific points.This shows electrons behave as particles:
They have mass and charge
Can be accelerated by voltage
Cause localised impacts, not spread-out wave effects
Supports the particle nature of electrons.
de Broglie's wavelength equation
λ=h/mv
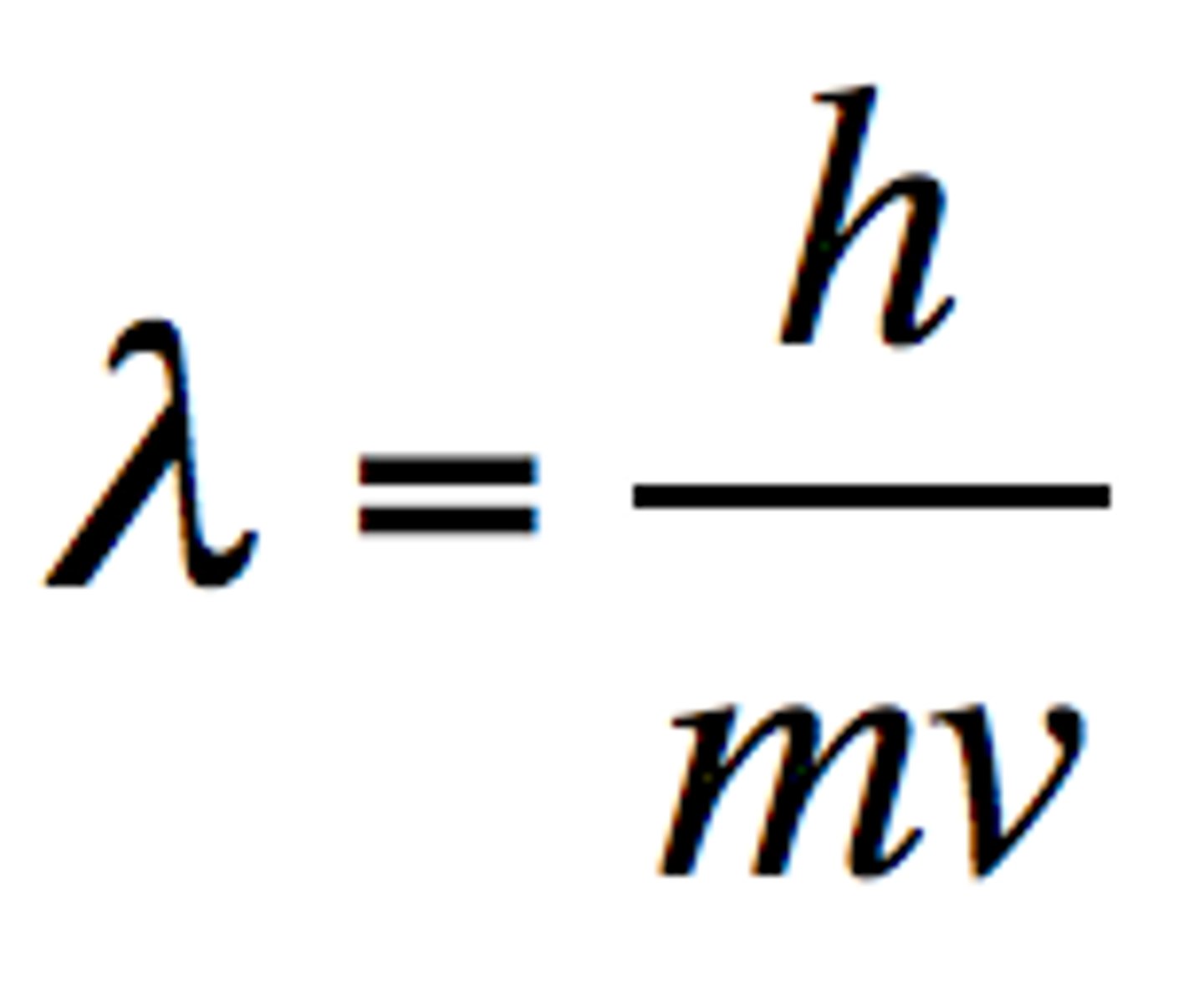
Limits on Observing Quantum Effects
Particles do not demonstrate measurable wave effects because their de Broglie wavelengths are extremely small.
For diffraction to occur, the particle must interact with an object around the same size as its wavelength. The gap needed to record diffraction in observable objects is often too small (smaller than atomic nuclei)