Core Practical 4: Investigate the hydrolysis of halogenoalkanes
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
Overview:

Procedure (Part 1):

Procedure (Part 2):

What is the pattern shown in part 1? (Cl vs Br vs I)
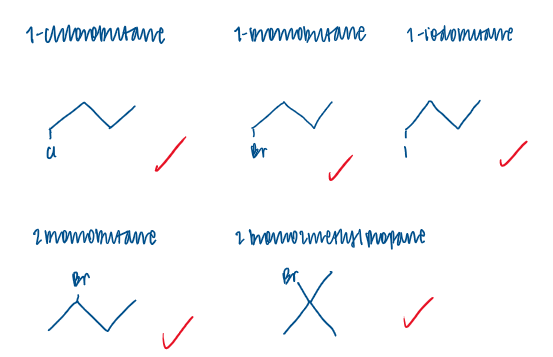
1-chlorobutane took the longest time to react with silver nitrate to form a silver chloride ppt.
1-iodobutane took the shortest time to react with silver nitrate and for a silver iodide ppt
1-chlorobutane> 1-bromobutane> 1-iodobutane
What is the pattern shown in part 2? (primary vs secondary vs tertiary)
tertiary haloalkane reacted the fastest to form a ppt
then the secondary haloalkane
then the primary haloalkane took the longest to react and form a ppt
A student suggested extending her investigation by repeating the process using 1-chlorobutane, 2-bromobutane and 2-iodo-2-methylpropane. Comment on this suggestion.
not good investigation
different structures and different halogens
cannot conclude that either the structure of the haloalkanes or the halogen in the haloalkane is contributing to the results of the investigation
only a single variable should be changed not both
Write an equation for the reaction of 1-bromobutane with water.
C4H9Br + H2O → C4H9OH + Br- + H+
In these reactions, a ppt forms.
Identify the ppt formed when the halogenoalkane is 1-iodobutane.
Silver Iodide (AgI)
Write an ionic equation for the formation of this ppt.
I- (aq) + Ag+ (aq) → AgI (s)
Explain why ethanol is used in these reactions.
ethanol has both polar and non-polar characteristics
polar - hydroxyl group
rest of molecule is alkyl chain
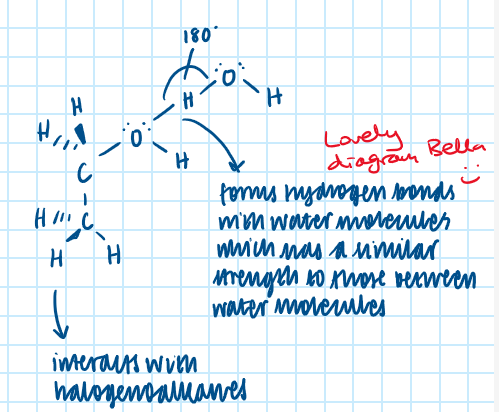
Explain why water is able to act as a nucleophile.
Remember - nucleophile is a species which seeks a positively charged area, and has a lone pair of electrons
Water has two lone pairs of electrons in each molecule.
This means that water can be an electron pair donor that seeks a positively charged area.
Explain why water is used as the nucleophile rather than hydroxide ions.
So that AgOH is not produced which would give an instant white ppt.
Draw skeletal formulae for each of the haloalkanes used in this investigation.