WJEC AS Chemistry Unit 1.6 - The Periodic Table
1/30
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Structure of the Periodic Table
Elements arranged according to increasing atomic number
Vertical columns; groups. All elements contain same outer electron configuration. Groups number; number of electrons in outer shell, therefore elements within a group have similar chemical properties
Horizontal rows; periods. All elements in a period have same number of quantum shells containing electrons
Table is divided into blocks; Gps 1 and 2=s-block as elements’s outer electrons are in an s orbital. Groups 3-8=in p-block. Elements between 2+3=in the d-block
Usually metals ok left and middle and non-metals on right
Oxidation and Reduction
gain oxygen=oxidised
Lost oxygen=reduced
Electron transfer; oxidation is loss of electrons, reduction is gain of electrons. Explained using half equations. Oxidising agent oxidised other reaction and is itself reduced. Reducing agent reduces other reactant and is itself oxidised.
Work out oxidation numbers of atoms/ions; increases=oxidised, decreases=reduced
Trends in ionisation energy
General increase across a period; increase in nuclear charge in same energy level so little extra shielding and therefore a greater attraction between nucleus and outer electrons
Decrease between Group 2 and Group 3; G3 elements’ outer electrons is in a new sub shell of slightly higher energy level and partly shielded by the s electrons
Decrease between G5 and G6; in G6, the electron is removed from an orbital containing a pair of electrons. The repulsion between these electrons makes the electron easier to remove. In G5, the electron is removed from a singly occupied orbital
Decrease down a group; outer electron has increased shielding from inner electrons and is further from nucleus. This outweighs increase in nuclear charge
Trends in electronegativity
Increases across a period; increase in nuclear charge but bonding electrons are always shielded by same inner electrons so there is a greater attraction between the nucleus and the bonding pair
Decrease down the group; bonding electrons have increased shielding from the nucleus, so attraction between nucleus and bonding electrons decreases
Most en elements at top right
Least en elements on bottom left
Trends in melting and boiling temperatures
decrease down group 1; increasing size of ions, strength of metallic bonding weakens, reducing energy required to break bonds and melt the metal
Decrease up group 7; decreasing strength of VdW as size decreases
Increase up to group 4 and decrease down to group 7; changes in electronegativity and strength of intermolecular forces. 1-4 en increases meaning atoms more likely to attract electrons and form stronger VdW bonds. 4-7: increased electronegativity and the strength of the chemical bonds within the molecules start to offset the effect of the stronger van der Waals forces
Reactions of Group 1 elements with water/steam
React vigorously with cold water to form the hydroxide and hydrogen
Reaction increases in vigour as you go down the group;
Lithium floats on water, gently fizzing
Sodium melts into a ball that dashes around the surface
Potassium melts into a ball and catches fire
Caesium explodes and shatters the glass container
Reactions of Group 1 elements with oxygen
form solid white oxides
Burn with a characteristic flame
Reactions of Group 2 elements with water/steam
react less vigorously than G2
The hydroxide and hydrogen are formed
Magnesium reacts very slowly
Reactivity increases as you go down the group
Calcium produced a steady stream of bubbles and the liquid becomes cloudy as a white precipitate of calcium hydroxide forms
Barium produces greater effervesce and the solution is clearer as barium hydroxide is more soluble
Reactions of Group 2 elements with Oxygen
Apart from Mg, all G2 metals tend to burn with a characteristic flame
All burn to form solid white oxides
Reactions of Group 1 elements with acids
too reactive to be added directly to acids
Reactions of Group 2 elements with acids
All G2 metals react vigorously with HCl to produce a colourless solution of the metal oxide and bubbles of hydrogen
Reactivity increases down the group
Only Mg reacts with sulfuric acid as others have insoluble sulfates
Reactions of aqueous cations
Characteristic flame colours of s-block cations
All s-block elements apart from Mg may be identified by a flame test
A clean metal wire/splint is moistened with HCl, dipped in the compound and held in a non-luminous Bunsen flame
Li+ = red
Na+ = orange-yellow
K+ = lilac
Mg2+ = (no colour)
Ca2+ = brick red
Sr2+ = crimson
Ba2+ = apple green
Trend in reactivity of Group 1 metals
Increases down the group due to increased atomic radius and decreased forces of electrostatic attratction ebtween electrons and nucelus
Trend in reactivity of Group 2 metals
Increases down the group due to decrease in IE and increase in shielding
Trend in thermal stability of Group 2 carbonates
All G2 carbonates decompose on heating to the oxide and carbon dioxide
Thermal stability increases down the group
Shown by heating the carbonate and seeing how long it take the carbon dioxide to turn the limewater cloudy
Trend in thermal stability of Group 2 hydroxides
All G2 hydroxides decompose on heating to the oxide and steam
The thermal stability decreases down the group
The hydroxides have to be heated more strongly before they will decompose
Trends in solubility in water of Group 1 compounds
All group 1 compounds are soluble
Trends in solubility in water of Group 2 compounds
many G2 compounds are in soluble
All nitrates are soluble
All carbonates are insoluble
Hydroxides become more soluble down the group
Sulfate become less soluble down the group
Basic characteristics of the oxides of Group 1
General; metal oxides are basic and non-metal oxides are acidic. All s-block metal oxides are strong basic and neutralise acids to form a salt and water
G1 oxides and barium oxide react with water to form a soluble hydroxide
The hydroxides are soluble so are alkalis
Basic characteristics of the hydroxides of Group 1
Formed when G1 oxides or barium oxide react with water to form a soluble hydroxide
Hydroxides are soluble so = alkalis
Basic characteristics of the oxides of Group 2
General; metal oxides are basic
All s-block metal oxides are strong bases
Neutralise acids to form a salt and water
Basic characteristics of the hydroxides of Group 2
Barium oxide and water forms a soluble hydroxide. Since hydroxide = soluble, they’re alkali
Other G2 hydroxides are not very soluble so saturated solutions of these hydroxides are only weakly basic as conc of hydroxide ion is very low
Trend in volatility of Group 7
decreases down the group
Number of electrons increases with atomic number, there’s an increase in the induced dipole-induced dipole intermolecular forces folding the diatomic molecule together
Melting and boiling temps increase down the group
Volatile; substances that form vapours easily
Substance with low bt has high volatility, high bt has low volatility
Reactions of the halogens with metals
React directly with most metals to form the halide
Trend in reactivity of the halogens in terms of relative oxidising power
Both reactivity and oxidising power decrease down the group
Halogens react by gaining electrons to form negative halide ions. Gain electrons during reactions so are reduced and oxidise the here substance
Down the group, the outer electrons are shielded more and are further from the nucleus
Harder to attract electrons
Test for halide ions
Silver nitrate test
The test has to be done in solution. If start with solid, must first dissolve in water
Add a few drops of nitric acid to neutralise and ensure any other anions are removed as they would also form precipitates
Aqueous silver nitrate solution is added to give;
Cl-; White precipitate
Br-; Cream precipitate
I-; (pale) Yellow precipitate
Precipitate formed is the insoluble silver halide
Difficult to differentiate between colours of precipitates in a single test where only one precipitate is seen
Aqueous ammonia is added to the precipitate;
AgCl; precipitate dissolves in dilute ammonia
AgBr; Precipitate does not dissolve much in dilute ammonia but dissolves in conc ammonia
AgI; Precipitate insoluble in dilute and conc ammonia
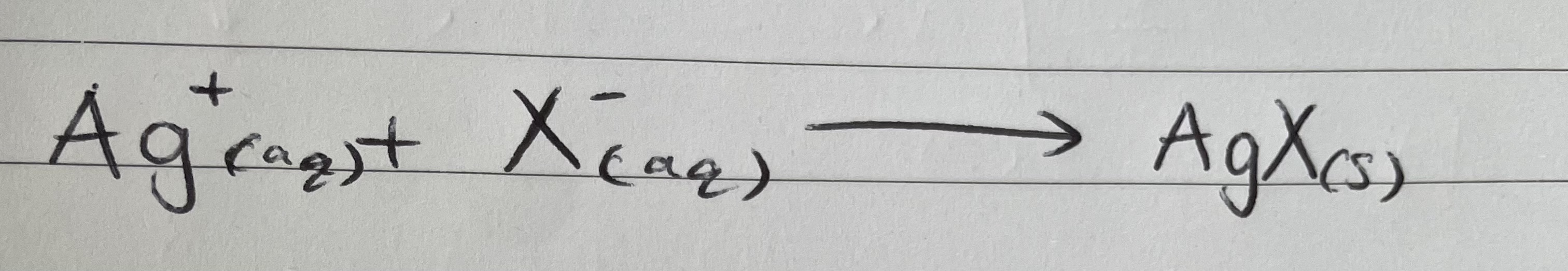
Displacement reactions of halogens
A halogen in a higher position in the group will oxidise a halide ion from lower in the group as oxidising powers decrease down the group
When a halogen is added to an aqueous solution containing a halide ion;
Chlorine displaces bromide and iodide
Bromine displaces only iodide
Iodine does not displace either chloride or bromide
When these displacement reactions happen, there are colour changes
Use of chlorine in water treatment and the related health and ethical issues
Chlorine is commonly added to water as the gaseous element and the equilibrium is established
The chlorate ion, ClO-, kills bacteria and other microbes so adding chlorine to water makes it safe to drink/swim in
Chlorination is used to prevent the outbreak of serious diseases
Risks;
Highly toxic
Can react with naturally occurring organic compounds found in the water supply to form chlorinated hydrocarbons which can cause liver and kidney cancer
Risks are small compared to risks of untreated water
Appear to be only beneficial effects below 1ppm
Some people object to water chlorination as forced mass medication
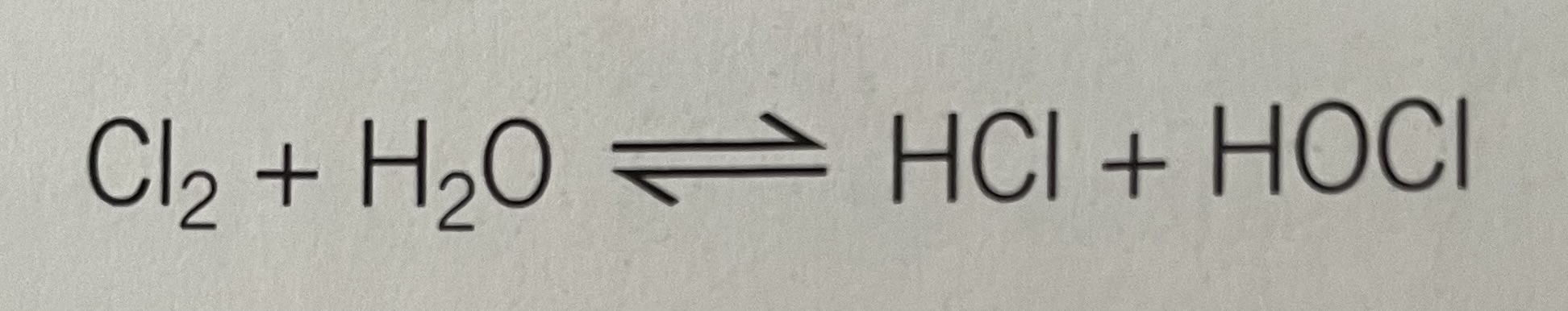
Use of fluoride ions in water treatment and the related health and ethical issue
Generally added to water to reduce tooth decay by preventing cavities
Water fluoridation reduces cavities in children
Effectiveness in adults is less clear
Can cause dental fluorosis which leads to tooth discolouration
No clear evidence of other adverse affects from water fluoridation
Appears to only have beneficial affects below 1ppm
Many people invested to water fluoridation as forced mass medication
Given the Prevalence in dental products - many people think adding fluoride to water supplies/bottled water can be detrimental to long-term dental health
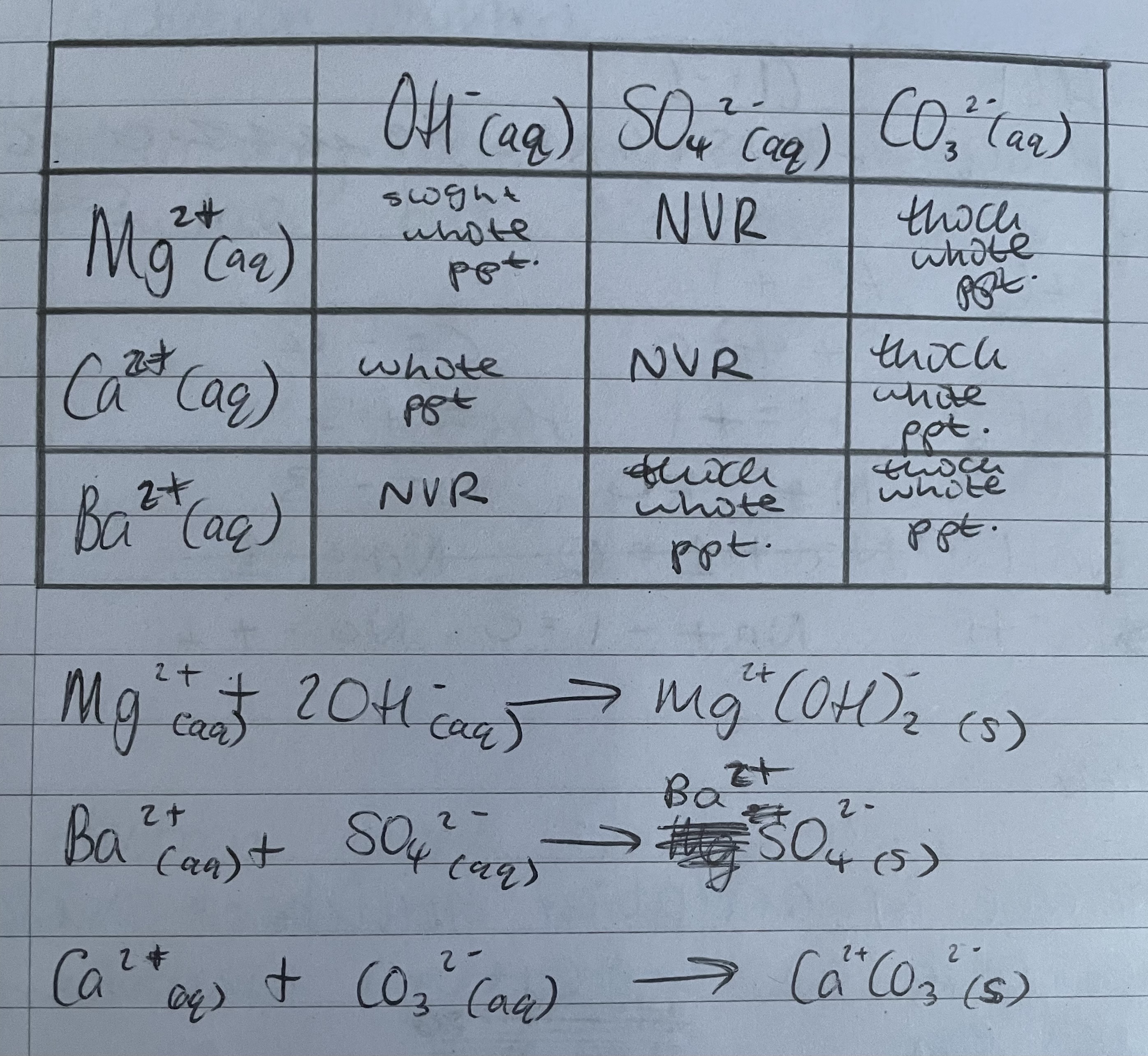
A solution is suspected to contain CO32-(aq), SO42-(aq) and OH-(aq). Describe the actions you would take to confirm this.
Carbonate; add a G2M salt and effervesence observed
Sulfate; add barium and a thick white ppt is formed
Hydroxide; add magnesium or calcium and a thin white ppt formed
Add an acid (nitric acid) and observe if an effervsence occurs. If carbonate ions are present, effervesecence will occur due to production of Carbon Dioxide; CO32-(aq) + H+(aq) →H2O + CO2(g)
Until effervesence occurs, add carbonate ions removed from solution
Divide solution in half
Add aqueuous barium ions (e.g. barium nitrate) to one half of the solution. Oberseve if a thick white ppt is formed.