CGIER 5 - Osmosis, Osmotic equilibrium, hemolysis & crenation
0.0(0)
Card Sorting
1/20
Earn XP
Description and Tags
Last updated 5:06 PM on 12/8/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
1
New cards
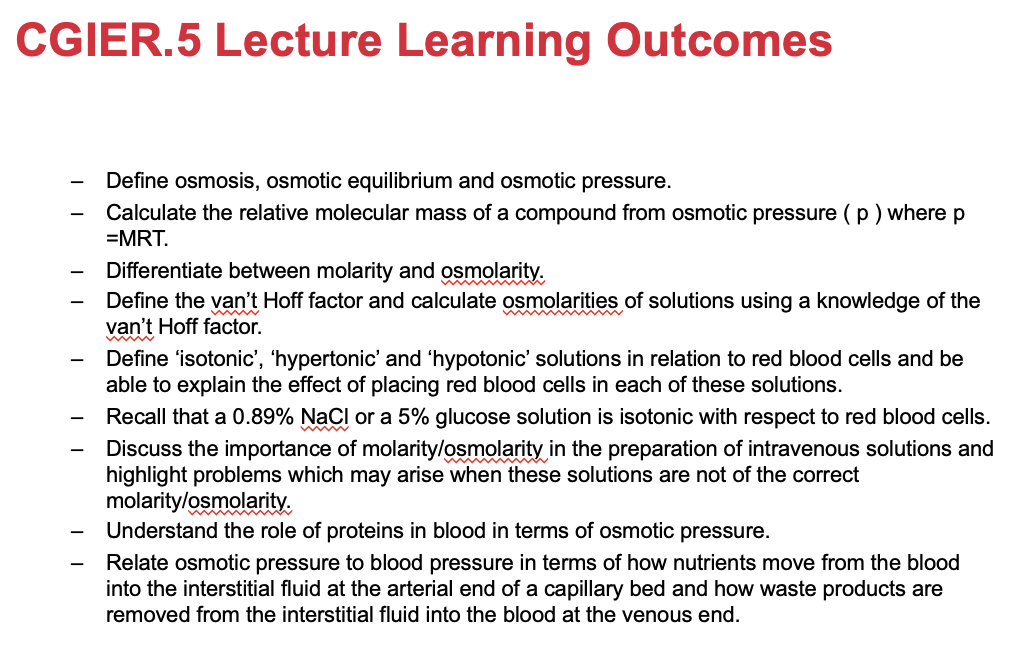
The passage of water in and out of living cells is an important biological process.
A semipermeable membrane is a material containing a network of microscopic holes or pores that permits the flow of small solvent molecules but restricts the flow of larger solute molecules
Solute – the substance being dissolved
Solvent – the substance “doing” the dissolving
A semipermeable membrane is a material containing a network of microscopic holes or pores that permits the flow of small solvent molecules but restricts the flow of larger solute molecules
Solute – the substance being dissolved
Solvent – the substance “doing” the dissolving
2
New cards
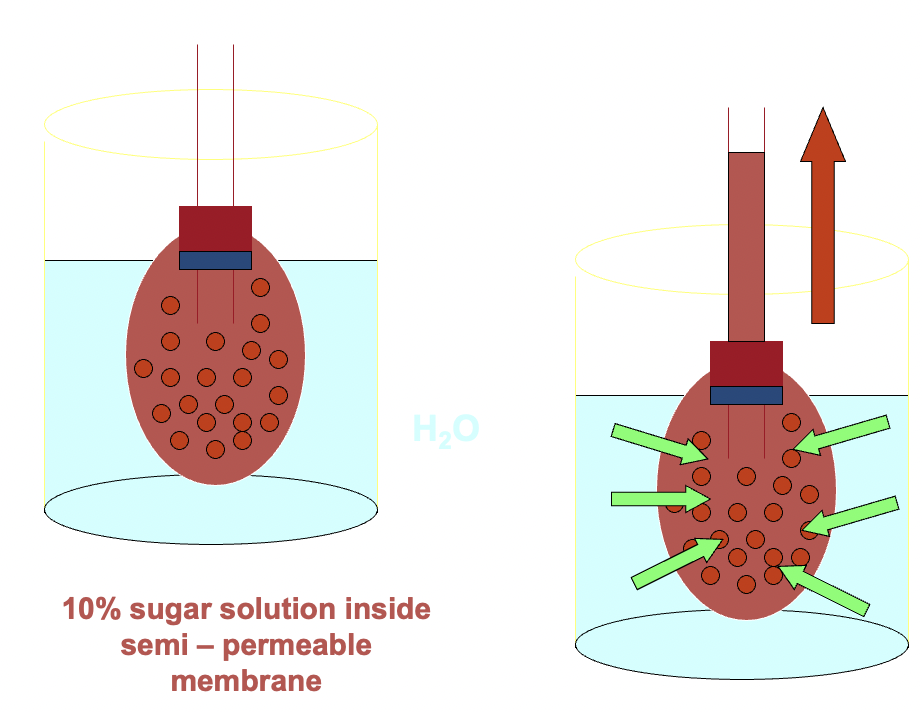
what's osmosis,
what's osmotic pressure
what's osmotic pressure
Osmosis is the *flow* of *solvent molecules through a semi-permeable membrane*
*from a region of lower solute concentration to a region of higher solute concentration*
e.g. if pure water and a sugar solution are separated by a semi-permeable membrane, water molecules will flow into the sugar solution at a higher rate than they return.
KEY WORDS EXAM:
* Semi-permeable membrane
* Movement of SOLVENT
* Region of lower SOLUTE concentration to region of higher SOLUTE concentration
diagram is showing green pressure arrows pushing solvent to region of power pressure
osmotic pressure: THE PRESSURE REQUIRED TO PREVENT OSMOSIS, bc we can prevent/reduce osmosis, maintains an equilibrium with no net movement of solvent. its *a measure of concentration*
*from a region of lower solute concentration to a region of higher solute concentration*
e.g. if pure water and a sugar solution are separated by a semi-permeable membrane, water molecules will flow into the sugar solution at a higher rate than they return.
KEY WORDS EXAM:
* Semi-permeable membrane
* Movement of SOLVENT
* Region of lower SOLUTE concentration to region of higher SOLUTE concentration
diagram is showing green pressure arrows pushing solvent to region of power pressure
osmotic pressure: THE PRESSURE REQUIRED TO PREVENT OSMOSIS, bc we can prevent/reduce osmosis, maintains an equilibrium with no net movement of solvent. its *a measure of concentration*
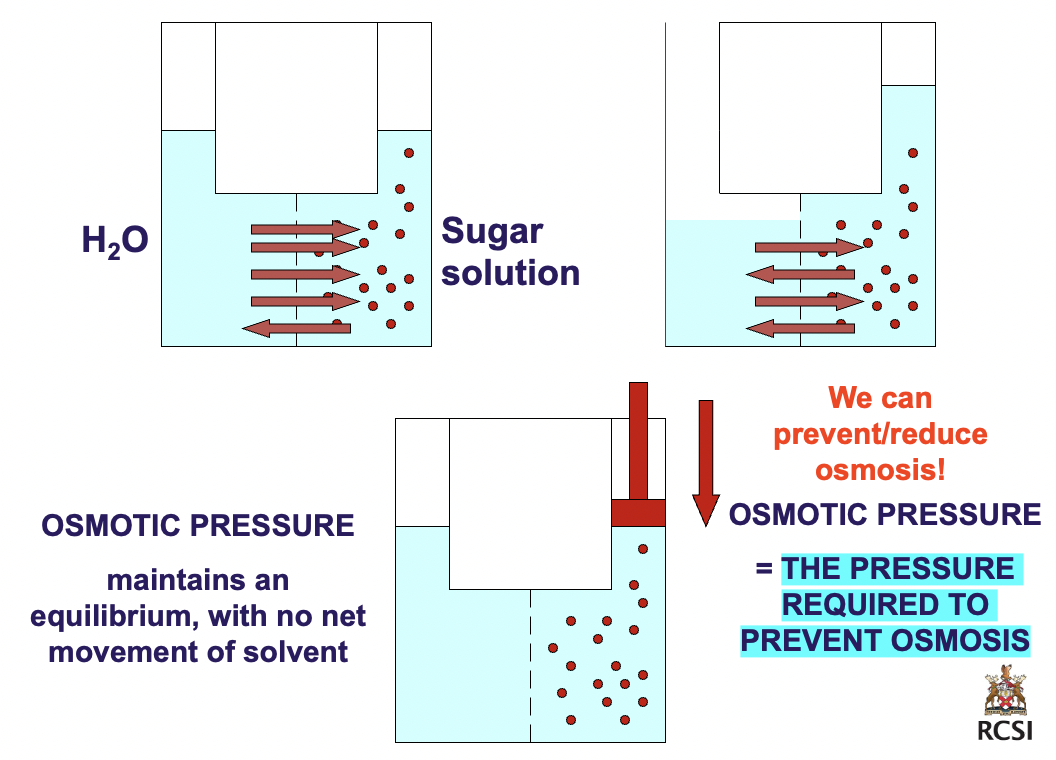
3
New cards
osmotic pressure (pulling power)
osmotic pressure: *exact* pressure necessary to prevent osmosis from taking place.
osmotic pressure (Π) proportionate to concentration concentration of particles
ΠV = nRT
Π = nRT/V
Π = MRT
M = molarity of solute
R = gas law constant
T = kelvin temperature
directly proportionate to [solute particles]
osmotic pressure (Π) proportionate to concentration concentration of particles
ΠV = nRT
Π = nRT/V
Π = MRT
M = molarity of solute
R = gas law constant
T = kelvin temperature
directly proportionate to [solute particles]
![osmotic pressure: *exact* pressure necessary to prevent osmosis from taking place.
osmotic pressure (Π) proportionate to concentration concentration of particles
ΠV = nRT
Π = nRT/V
Π = MRT
M = molarity of solute
R = gas law constant
T = kelvin temperature
directly proportionate to [solute particles]](https://knowt-user-attachments.s3.amazonaws.com/beb90d75febc40b3b12c41d945fc15c9.jpeg)
4
New cards
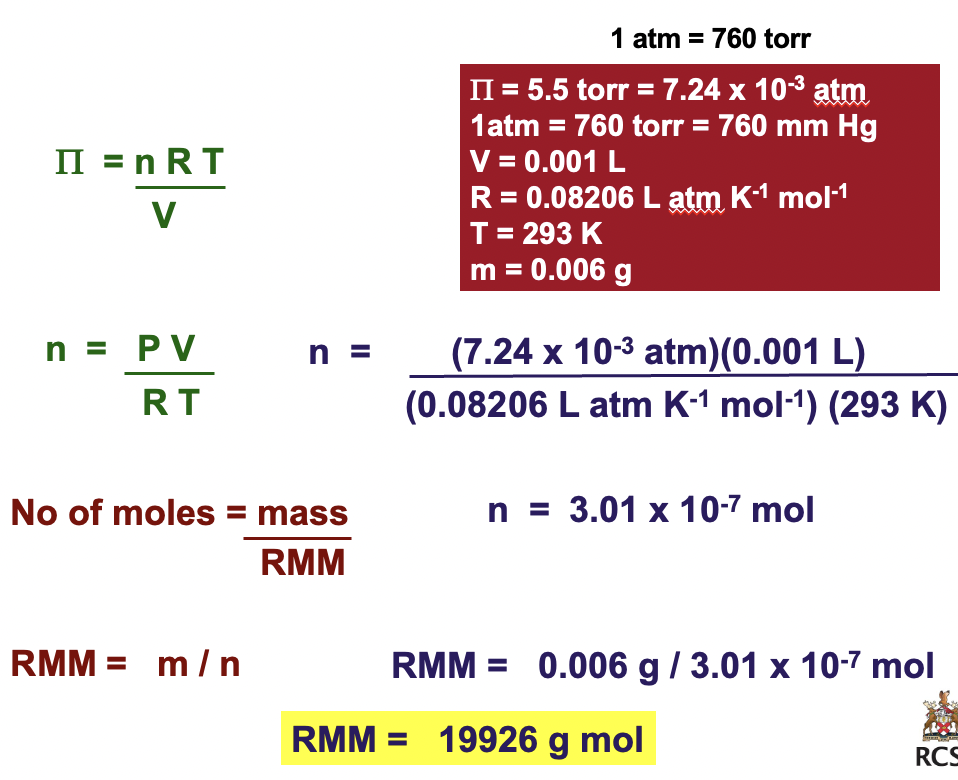
Calculate the RMM from Osmotic Pressure (pulling power)
A 6 mg sample of a protein is dissolved in water to give 1 ml of solution.
Calculate the RMM (relative molecular mass) of the protein if the osmotic pressure is 5.5 torr at 20 0C?
1 atm = 760 torr
A 6 mg sample of a protein is dissolved in water to give 1 ml of solution.
Calculate the RMM (relative molecular mass) of the protein if the osmotic pressure is 5.5 torr at 20 0C?
1 atm = 760 torr
Π = 5.5 torr = 7.24 x 10'-3 atm
1atm = 760 torr = 760 mm Hg
V = 0.001 L
R = 0.08206 L atm K'-1 mol'-1
T = 293 K
m = 0.006 g
Π = nRT/V
n = ΠV/RT
No of moles = mass/RMM
RMM = m/n
n = (7.24 x 10'-3 atm)(0.001L)/(0.08206 L atm K'-1 mol'-1)(293 K)
n = 3.01 x 10'-7 mol
RMM = 0.006g/3.01 x 10'-7 mol
RMM = 19926 g mol
common sense has to prevail in everything u do
1atm = 760 torr = 760 mm Hg
V = 0.001 L
R = 0.08206 L atm K'-1 mol'-1
T = 293 K
m = 0.006 g
Π = nRT/V
n = ΠV/RT
No of moles = mass/RMM
RMM = m/n
n = (7.24 x 10'-3 atm)(0.001L)/(0.08206 L atm K'-1 mol'-1)(293 K)
n = 3.01 x 10'-7 mol
RMM = 0.006g/3.01 x 10'-7 mol
RMM = 19926 g mol
common sense has to prevail in everything u do
5
New cards
Osmolarity vs Molarity
osmosis is proportional to concentration
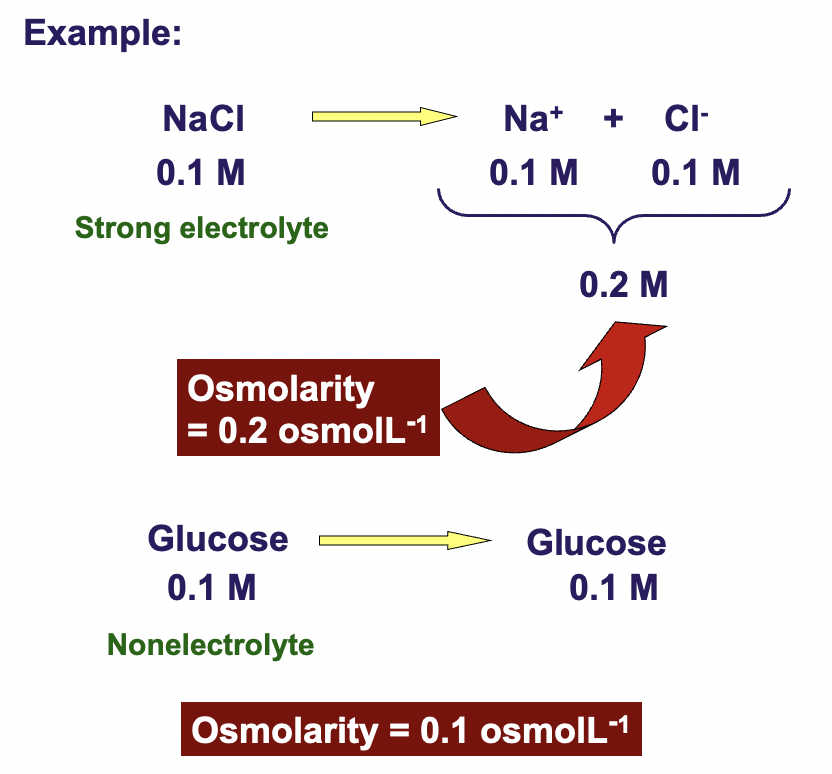
0.1 M NaCl
molarity of the solution doesn't reveal enough abt the solution in terms of osmotic pressure
to express the concentration of all the particles in the solution contributing to the osmotic pressure, medical scientists use 'OSMOLARITY'
the greater the molarity of solN, the greater the osmotic pressure
Greater # of particles = greater pulling power = greater osmotic pressure
Glucose has covalent molecules that dissolve but don't break up
0.1 M NaCl
molarity of the solution doesn't reveal enough abt the solution in terms of osmotic pressure
to express the concentration of all the particles in the solution contributing to the osmotic pressure, medical scientists use 'OSMOLARITY'
the greater the molarity of solN, the greater the osmotic pressure
Greater # of particles = greater pulling power = greater osmotic pressure
Glucose has covalent molecules that dissolve but don't break up
6
New cards
Osmolarity
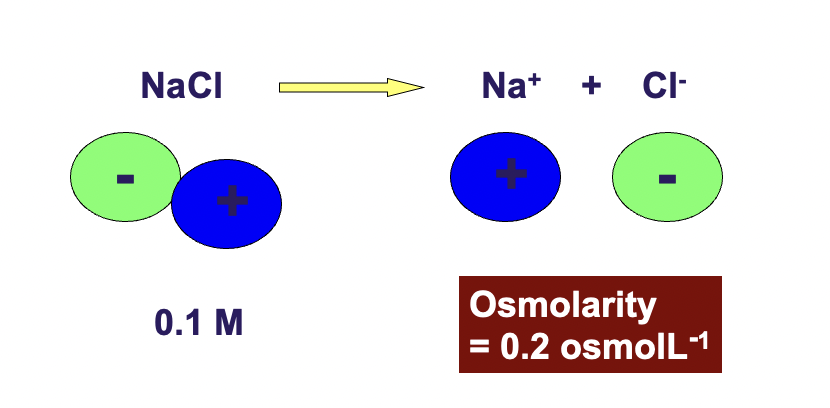
Solutions containing different particles but having the same osmolarity will have the same osmotic pressure
0.1 M NaCl = 0.2 M glucose
0.1 M NaCl is osmolar to 0.2 gluclose bc 0.1 NaCl will dissociate into 0.1 Na+ 0.1 Cl- = 0.2osmoles/litre
all ionic compounds dissociate into ions in solution which in turn increases the total number of particles in solution
0.1 M NaCl = 0.2 M glucose
0.1 M NaCl is osmolar to 0.2 gluclose bc 0.1 NaCl will dissociate into 0.1 Na+ 0.1 Cl- = 0.2osmoles/litre
all ionic compounds dissociate into ions in solution which in turn increases the total number of particles in solution
7
New cards
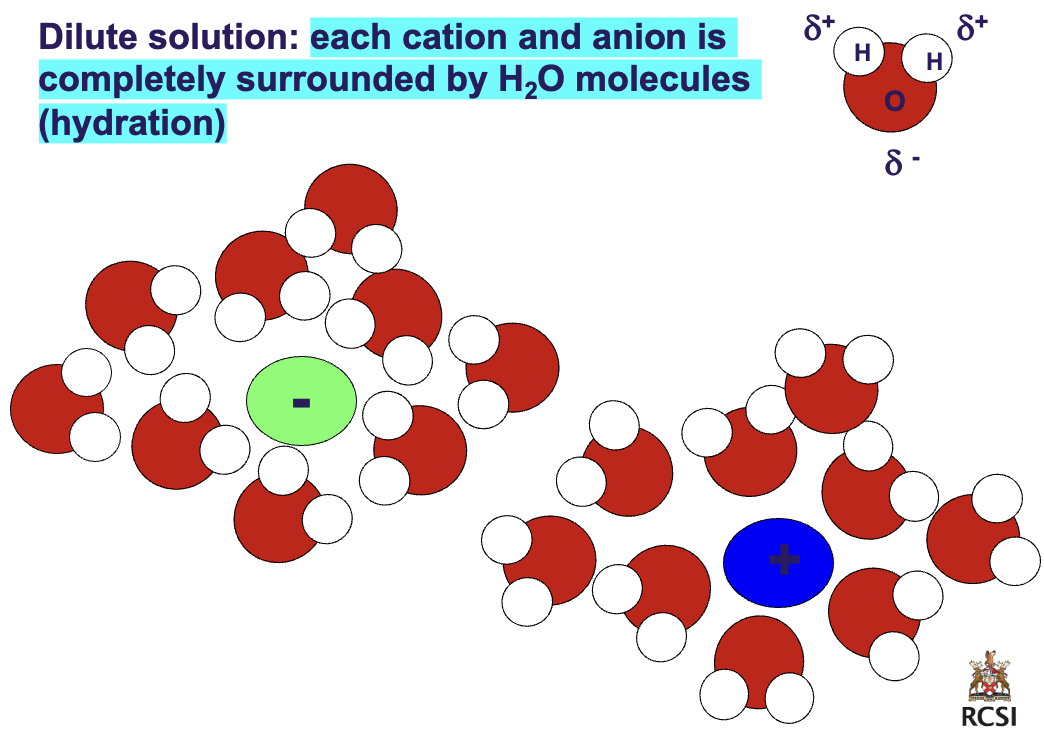
Dilute vs. concentrated solution
dilute: each cation and anion is completely surrounded by H2O molecules (hydration)
concentrated: each cation and anion have incomplete hydration and therefore tend to form *ion pairs*
> less water, more solute (NaCl) not enough water to complete hydration sphere around ions so u get ion Paris
concentrated: each cation and anion have incomplete hydration and therefore tend to form *ion pairs*
> less water, more solute (NaCl) not enough water to complete hydration sphere around ions so u get ion Paris
8
New cards

The van't Hoff factor (i)
Jacob's henries van't hoff, nobel in chem 1901
*EXAM MAY BE ASKED TO PREDICT VAN'T FACTOR*
Jacob's henries van't hoff, nobel in chem 1901
*EXAM MAY BE ASKED TO PREDICT VAN'T FACTOR*
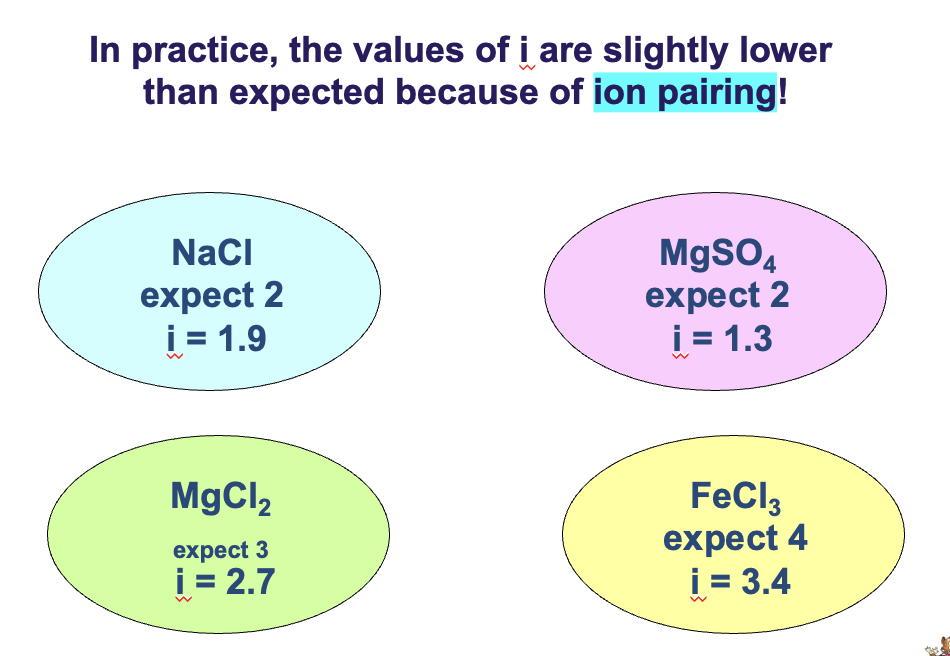
i = actual no. of particles in solution after dissociation (expect 2 for Na+ Cl-) / no. of formula units initially dissolved in solN (1 for every NaCl)
NaCl expect i = 2 but i = 1.9
MgCl2 expect i = 3 but i = 2.7
higher the charge greater the attraction, therefore the number is slightly lower
in practice the values of i are slightly lower than expected because of ION PAIRING!
NaCl expect i = 2 but i = 1.9
MgCl2 expect i = 3 but i = 2.7
higher the charge greater the attraction, therefore the number is slightly lower
in practice the values of i are slightly lower than expected because of ION PAIRING!
9
New cards
Calculate osmolarity
e.g. = 0.01 M NaCl
Molarity = 0.01 mol L'-1
BUT
i = 1.9
Osmolarity = i.M
e.g. = 0.01 M NaCl
Molarity = 0.01 mol L'-1
BUT
i = 1.9
Osmolarity = i.M
Therefore osmolarity = 1.9x0.01 = 0.019 osmolL'1
10
New cards
A comparison of the osmolarity of blood and urine is often a good indication of kidney function
Normal blood serum osmolarity is 280 - 290 mosmol/L,
and normal urine osmolarity is 500 - 800 mosmol/L.
Osmolarity is measure of osmotic pressure
and normal urine osmolarity is 500 - 800 mosmol/L.
Osmolarity is measure of osmotic pressure
11
New cards
hypertonic, isotonic, hypotonic
isotonic - a solution having the SAME osmotic pressure as blood, same [solute] as RBC
hypertonic - a solution having HIGHER osmotic pressure , higher [solute] than blood (RBC will burst)
hypotonic - a solution having LOWER osmotic pressure than blood, lower [solute] than blood (RBC will shrivel up)
hypertonic - a solution having HIGHER osmotic pressure , higher [solute] than blood (RBC will burst)
hypotonic - a solution having LOWER osmotic pressure than blood, lower [solute] than blood (RBC will shrivel up)
![isotonic - a solution having the SAME osmotic pressure as blood, same [solute] as RBC
hypertonic - a solution having HIGHER osmotic pressure , higher [solute] than blood (RBC will burst)
hypotonic - a solution having LOWER osmotic pressure than blood, lower [solute] than blood (RBC will shrivel up)](https://knowt-user-attachments.s3.amazonaws.com/b2e64b3b7f2c4c5b906709ec26bbbc93.jpeg)
12
New cards
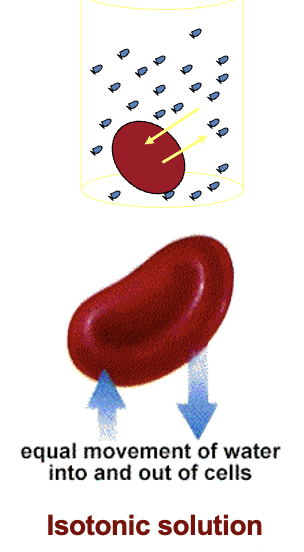
A normal saline solution
(0.9% NaCl solution)
is isotonic to red blood cells.
(0.9% NaCl solution)
is isotonic to red blood cells.
When red blood cells are
placed in a saline solution
no change will be observed.
SINCE NO DIFFERENCE IN OSMOLARITY
placed in a saline solution
no change will be observed.
SINCE NO DIFFERENCE IN OSMOLARITY
13
New cards
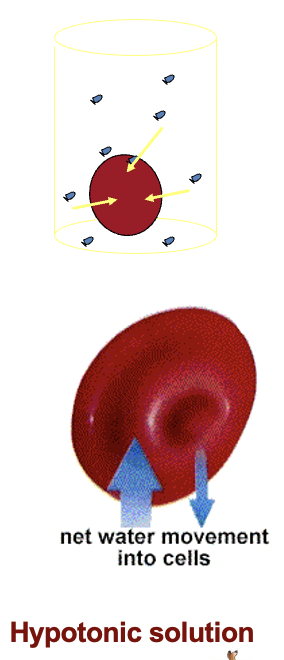
If red blood cells are placed in a solution of
lower solute concentration.
(explain Hemolysis)
lower solute concentration.
(explain Hemolysis)
i.e. a hypotonic solution, (e.g. distilled water)
water will move into the cells.
The cells will then swell until equilibrium is restored or rupture.
Haemolysis
water will move into the cells.
The cells will then swell until equilibrium is restored or rupture.
Haemolysis
14
New cards
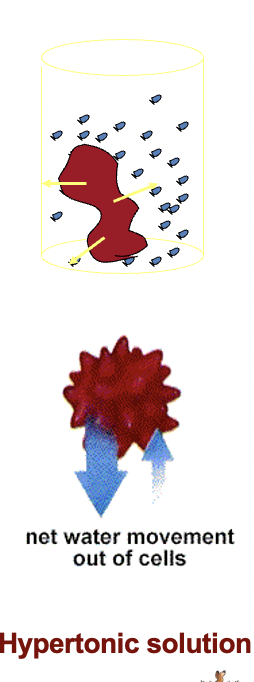
Explain crenation
If red blood cells are placed in a solution of higher concentration i.e. a hypertonic solution (e.g. 3% NaCl)
the water will move from the cells to
the surrounding solution.
The cells will shrink until equilibrium is restored.
the water will move from the cells to
the surrounding solution.
The cells will shrink until equilibrium is restored.
15
New cards
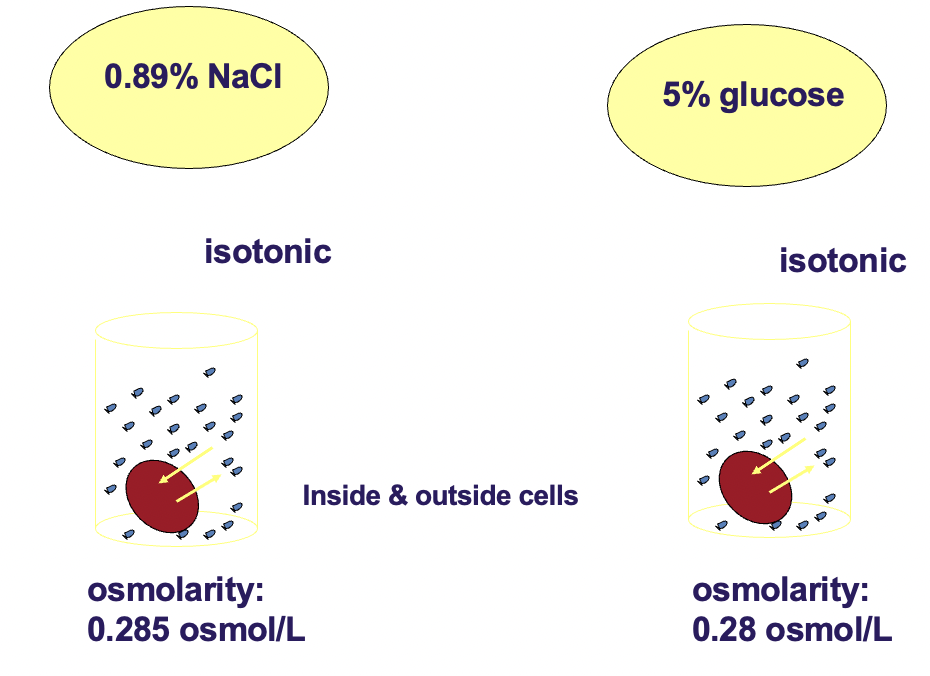
Isotonicity of NaCl & glucose solutions with respect to red blood cells!
0.89% NaCl is isotonic with respect to RBC
5% glucose is isotonic with respect to RBC
*solutions cannot be safely introduced into the bloodstream unless they are isotonic with blood. all intravenous preparations are made with isotonic solution*
5% glucose is isotonic with respect to RBC
*solutions cannot be safely introduced into the bloodstream unless they are isotonic with blood. all intravenous preparations are made with isotonic solution*
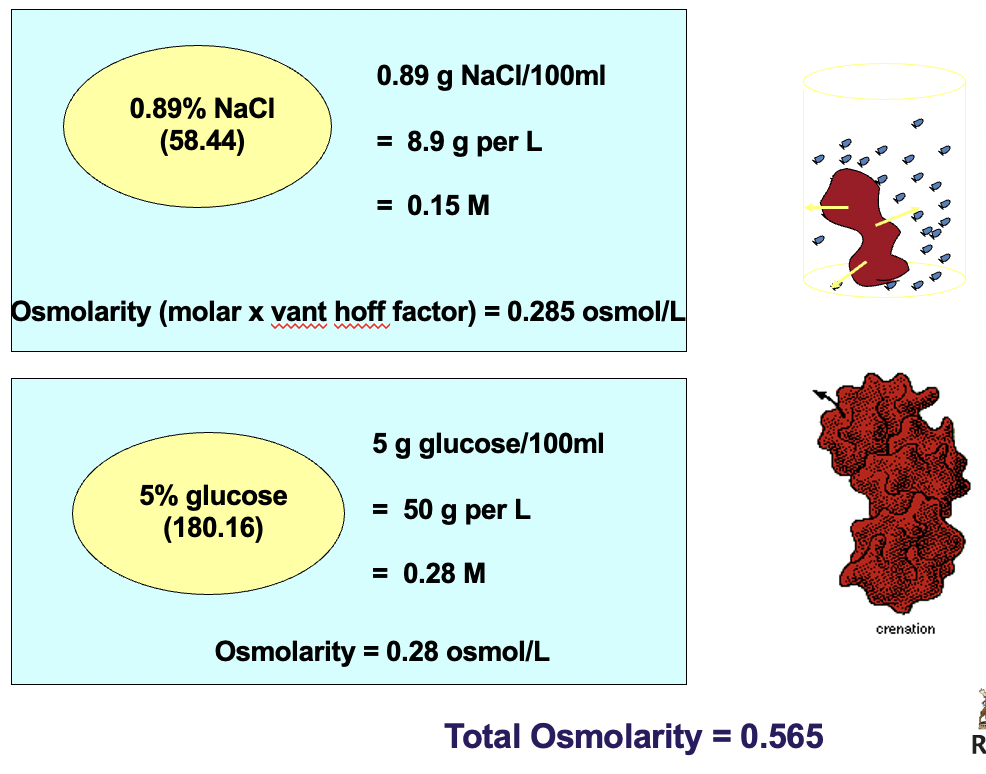
16
New cards
intravenous solutions, why use them?
Some danger in injecting fluids and electrolytes directly
into the blood stream
Why use IV fluids?
> Patients who can’t swallow safely
(coma, anaesthesia, have seizures, severe vomiting)
> Patients who can’t drink enough to keep up with their loss of fluids (major burns, haemorrage)
> Patients who require medication which is
destroyed by gastric juices
> Patients who must rapidly increase [medication]
or [electrolyte] in blood
into the blood stream
Why use IV fluids?
> Patients who can’t swallow safely
(coma, anaesthesia, have seizures, severe vomiting)
> Patients who can’t drink enough to keep up with their loss of fluids (major burns, haemorrage)
> Patients who require medication which is
destroyed by gastric juices
> Patients who must rapidly increase [medication]
or [electrolyte] in blood
17
New cards
Problems can occur when IV solutions are not isotonic
e.g.
a person who is badly dehydrated and needs increased H2O in the bloodstream:
option 1 = rapid IV infusion of pure H2O
option 2 = IV infusion of 5% dextrose
e.g.
a person who is badly dehydrated and needs increased H2O in the bloodstream:
option 1 = rapid IV infusion of pure H2O
option 2 = IV infusion of 5% dextrose
option 1:
> RBC and endothelial cells would swell
> rupture of the endothelial cells and hemolysis!
option 2:
> nearly isotonic w RBC
> easily tolerated by patient
> as dextrose metabolized, H2O remains
> RBC and endothelial cells would swell
> rupture of the endothelial cells and hemolysis!
option 2:
> nearly isotonic w RBC
> easily tolerated by patient
> as dextrose metabolized, H2O remains
18
New cards
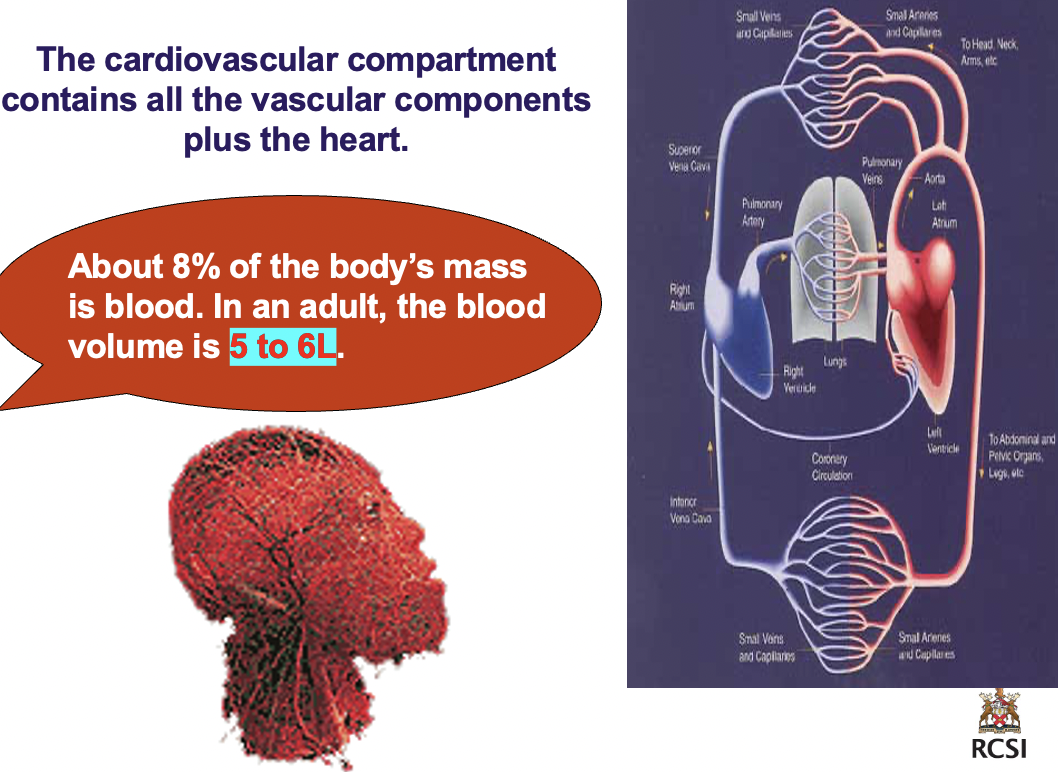
blood and the absorption of nutrients by cells
2 main lines of communication within the body are ciruclatory system and nervous system
cardiovascular compartment contains all the vascular components + the heart
> abt 8% of the body's mass is *blood*
> in an adult the blood volume is 5-6L
*PROTEINS IN BLOOD ARE VITAL TO OSMOTIC PRESSURE*
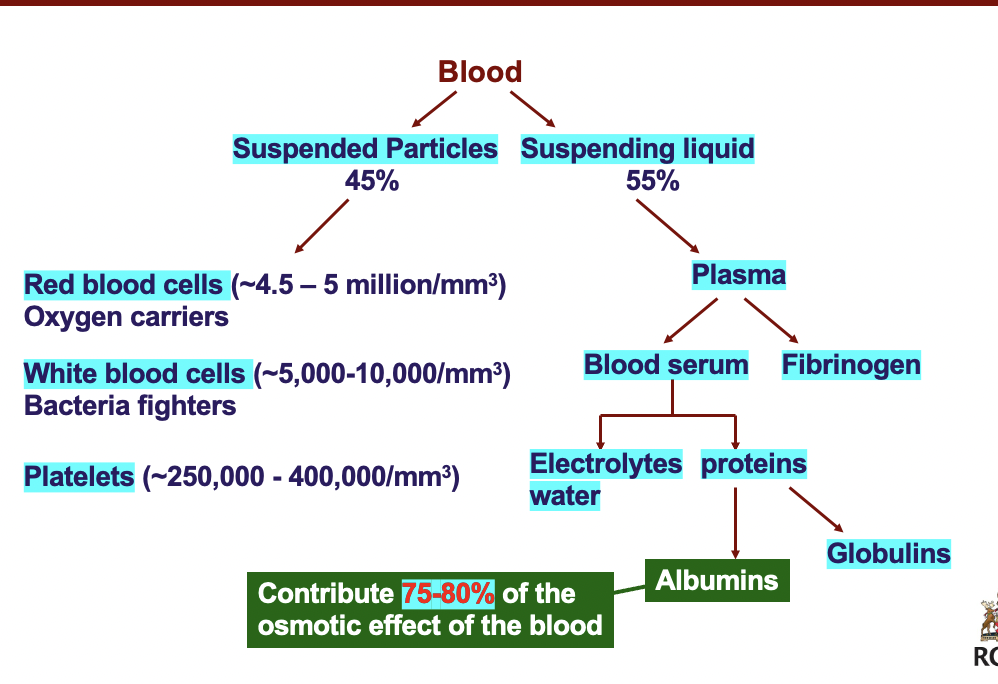
Blood:
> suspended particles: RBC, WBC, platelets
> suspending liquid: plasma: fibrinogen; blood serum: electrolytes, water ; proteins: globulins; albumins
*albumins contribute to 75-80% of the osmotic effect of the blood*
blood enters capillary bed as arterial blood but leaves on the other side as venous blood. during the switch, fluids and nutrients leave blood and move into interstitial fluid and then into tissue cells, fluids return to bloodstream in same volume but must carry waste products of metabolism.
rate of diffusion of fluids is ~25-30L per second
cardiovascular compartment contains all the vascular components + the heart
> abt 8% of the body's mass is *blood*
> in an adult the blood volume is 5-6L
*PROTEINS IN BLOOD ARE VITAL TO OSMOTIC PRESSURE*
Blood:
> suspended particles: RBC, WBC, platelets
> suspending liquid: plasma: fibrinogen; blood serum: electrolytes, water ; proteins: globulins; albumins
*albumins contribute to 75-80% of the osmotic effect of the blood*
blood enters capillary bed as arterial blood but leaves on the other side as venous blood. during the switch, fluids and nutrients leave blood and move into interstitial fluid and then into tissue cells, fluids return to bloodstream in same volume but must carry waste products of metabolism.
rate of diffusion of fluids is ~25-30L per second
19
New cards
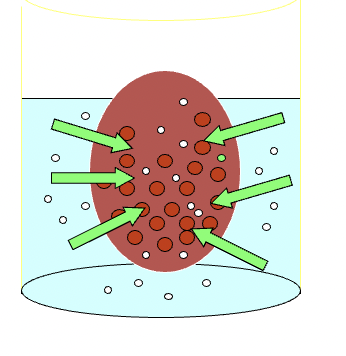
higher level of protein in blood relative to the ISF is the main reason why it has:
main reason why it has higher osmotic pressure
> nutrients and small ions in blood ya
> but there's LARGE proteins
bc of higher osmotic pressure, water and nutrients tend to move into the blood from the interstitial compartment
*this cannot be allowed to happen everywhere or the interstitial spaces would eventually become too dehydrated to maintain life.
> nutrients and small ions in blood ya
> but there's LARGE proteins
bc of higher osmotic pressure, water and nutrients tend to move into the blood from the interstitial compartment
*this cannot be allowed to happen everywhere or the interstitial spaces would eventually become too dehydrated to maintain life.
20
New cards
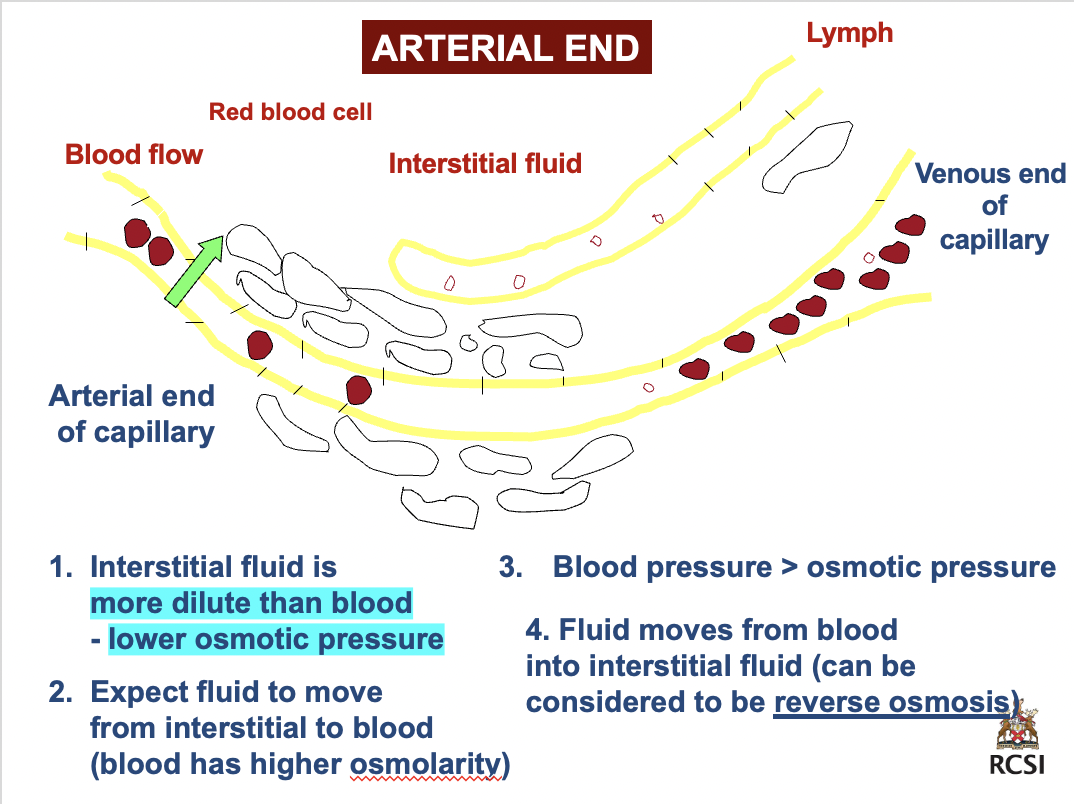
arterial end
+
venous end
+
venous end
arterial end
1. interstitial fluid is *more dilute than blood- lower osmotic pressure*
2. expect fluid to move from interstitial to blood (blood has higher osmolarity)
3. BP > osmotic pressure
4. fluid moves from blood into interstitial fluid (can be considered to be reverse osmosis)
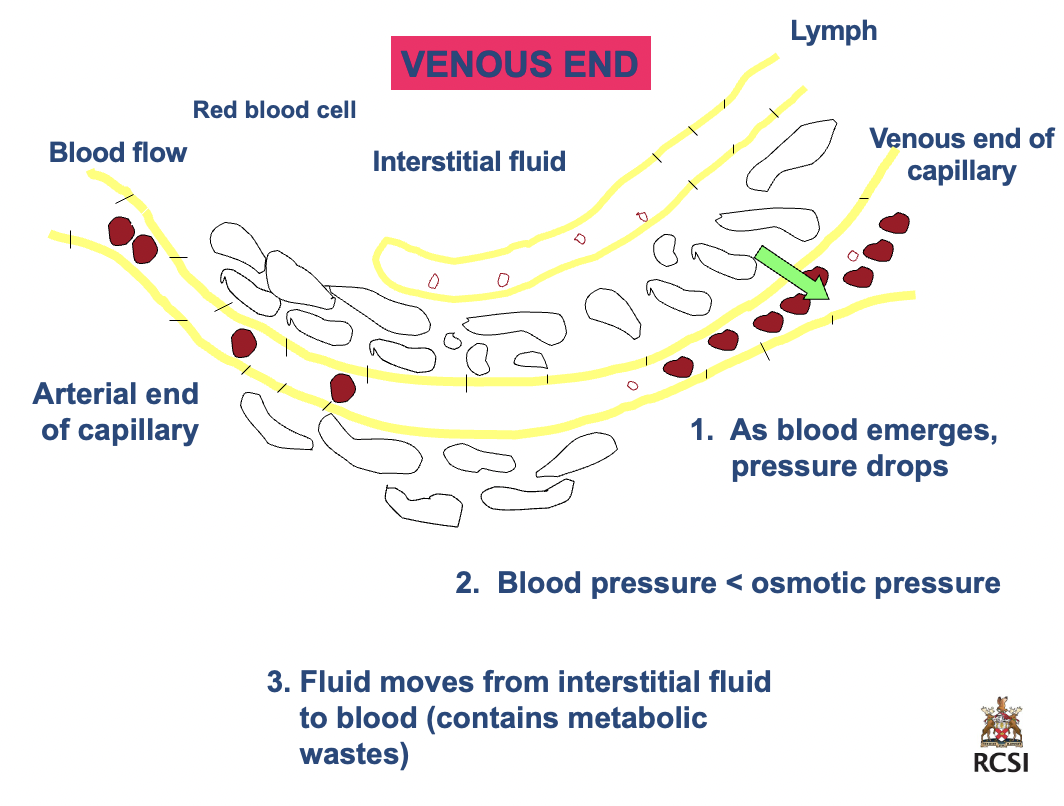
_____
venous end
1. as blood emerges, pressure drops
2. blood pressure < osmotic pressure
3. fluid moves from interstitial fluid to blood (contains metabolic wastes)
1. interstitial fluid is *more dilute than blood- lower osmotic pressure*
2. expect fluid to move from interstitial to blood (blood has higher osmolarity)
3. BP > osmotic pressure
4. fluid moves from blood into interstitial fluid (can be considered to be reverse osmosis)
_____
venous end
1. as blood emerges, pressure drops
2. blood pressure < osmotic pressure
3. fluid moves from interstitial fluid to blood (contains metabolic wastes)
21
New cards
summaries
arterial end:
> BP > Osmotic pressure
> net movement into interstitial fluids (oxygen)
venous end:
> BP < Osmotic pressure
> net movement into bloodstream (trash and CO2)
> BP > Osmotic pressure
> net movement into interstitial fluids (oxygen)
venous end:
> BP < Osmotic pressure
> net movement into bloodstream (trash and CO2)