Topic 15: Reactions to rememember
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
What are the different types of reactions that transition metal complexes can have?
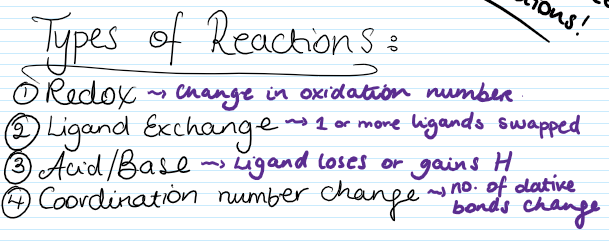
When you oxidise a metal, what conditions do you need?
Alkaline conditions
When you reduce a metal, what conditions do you need?
Acidic conditions
If you want a transition metal-aqua ion, what would you need to do to the transition metal ion?

Give the general reaction for a hexaqua metal complex and chloride ions

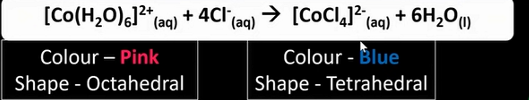
What is the reaction between [Co(H2O)6]2+ and chloride ions? State any colours
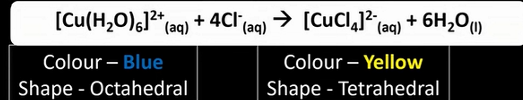
What is the reaction between [Cu(H2O)6]2+ and chloride ions? State any colours
What is the reaction between [Fe(H2O)6]3+ and chloride ions? State any colours

When transition metal aqua complexes react with OH ions, what can be said about the pattern between charge and OH ions susbtituted?
The number of OH- substituted is same as value of charge on the initial ion.
What is the reaction between [Cr(H2O)6]3+ and NaOH (OH ions)? State any colours
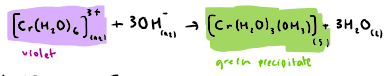
What is the reaction between [Fe(H2O)6]2+ and NaOH (OH ions)? State any colours
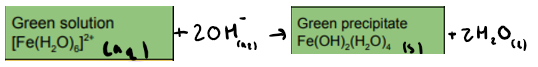
What is the reaction between [Fe(H2O)6]3+ and NaOH (OH ions)? State any colours

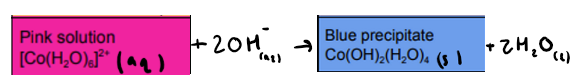
What is the reaction between [Co(H2O)6]2+ and NaOH (OH ions)? State any colours
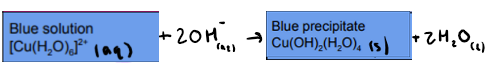
What is the reaction between [Cu(H2O)6]2+ and NaOH (OH ions)? State any colours
Only one transition metal can reach with excess OH ions, which transition metal-aqua ion is this? Also include reactions
Chromium [Cr(H2O)6]3+
![<p>Chromium [Cr(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/e48e5293-52de-4f92-adf4-eaa713832801.png)
Why can other transition metal-aqua ions not react with excess OH-?
They are insoluble in excess NaOH (their colour will be same as colour with same amount of OH ion)
When transition metal aqua complexes react with ammonia, what can be said about the pattern between charge and NH3 ions susbtituted?
The number of NH3 substituted is same as value of charge on the initial ion.
When transition metal aqua complexes react with ammonia, what can be said about the pattern between number of NH3 reactants and number of NH4+ produced?

What is the reaction between [Cr(H2O)6]3+ and ammonia? State any colours

What is the reaction between [Fe(H2O)6]2+ and ammonia? State any colours

What is the reaction between [Fe(H2O)6]3+ and ammonia? State any colours
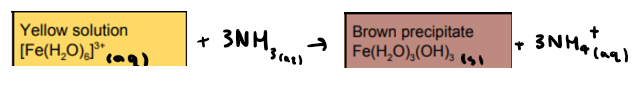
What is the reaction between [Co(H2O)6]2+ and ammonia? State any colours
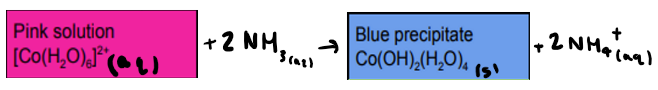
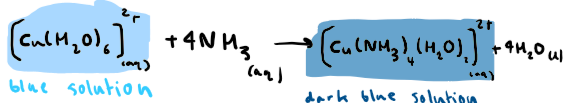
What is the reaction between [Cu(H2O)6]2+ and ammonia? State any colours

Which transition metal-aqua ions can react with excess ammonia?
[Cr(H2O)6]3+
[Co(H2O)6]2+
[Cu(H2O)6]2+
Why can other transition metal-aqua ions not react with excess ammonia?
They are insoluble in excess ammonia (their colour will be same as colour with same amount of ammonia)
What is the reaction between [Cr(H2O)6]3+ and excess ammonia? State any colours
Or [Cr(H2O)6]3+ (violet solution, aq)+ 6NH3 (aq)→ [Cr(NH3)6]3+ (purple solution, aq) + 6H2O (l)
![<p>Or [Cr(H<sub>2</sub>O)<sub>6</sub>]<sup>3+ </sup>(violet solution, aq)+ 6NH<sub>3</sub> (aq)→ [Cr(NH3)<sub>6</sub>]<sup>3+ </sup>(purple solution, aq) + 6H<sub>2</sub>O (l)</p>](https://knowt-user-attachments.s3.amazonaws.com/38d5043a-5f95-4123-a58e-240a8482250b.png)
What is the reaction between [Co(H2O)6]2+ and excess ammonia? State any colours

What is the reaction between [Cu(H2O)6]2+ and excess ammonia? State any colours