Reduction
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
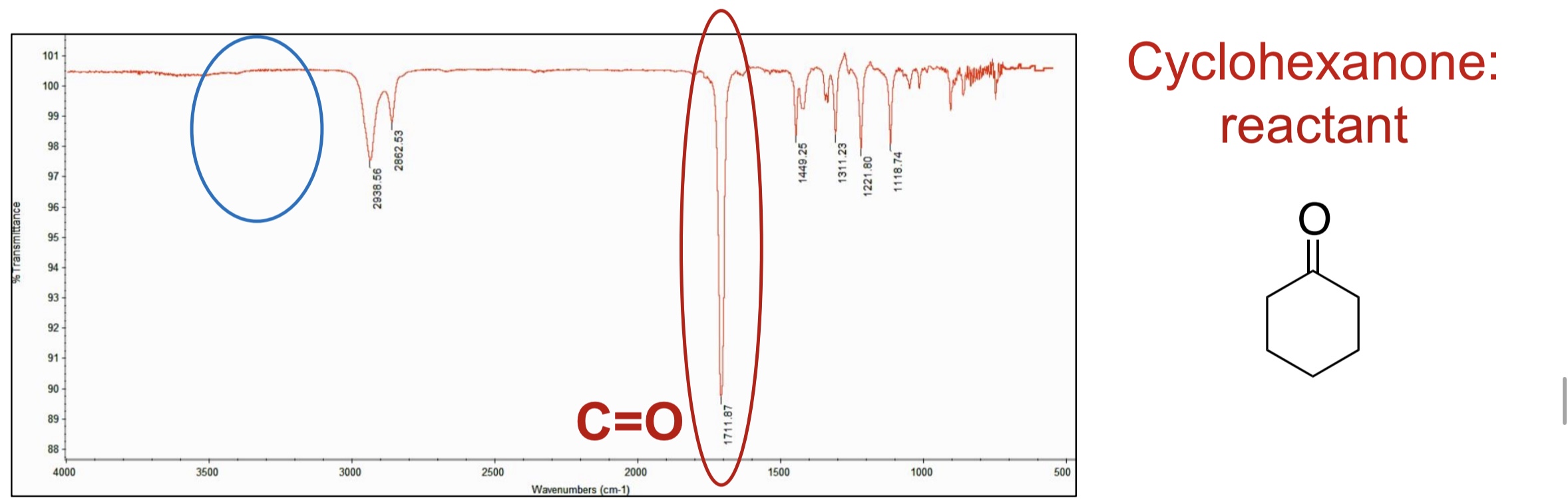
What peak is present in the beggining of the starting material that will disappear?
C=O
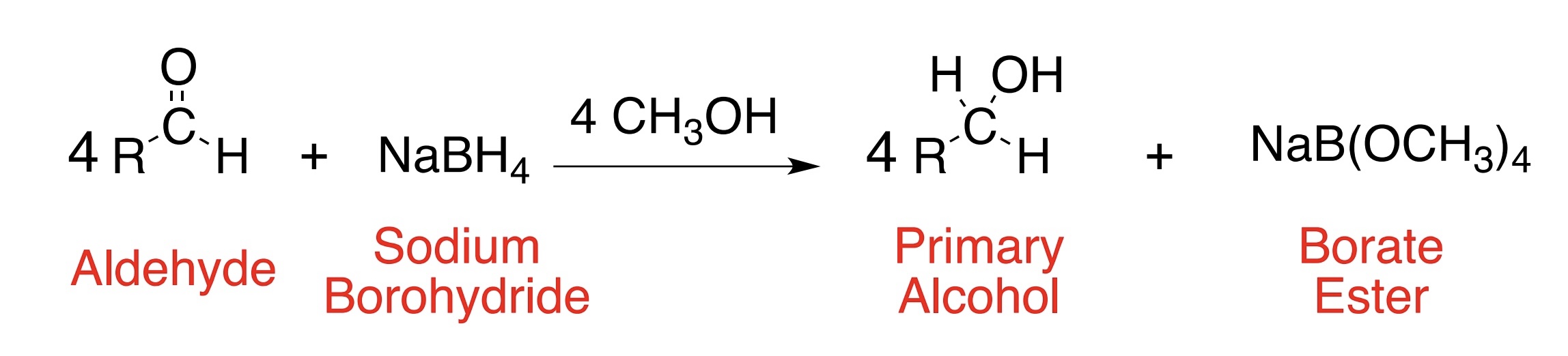
What is the product of reduction of an aldehyde using NaBH4?
Primary alcohol
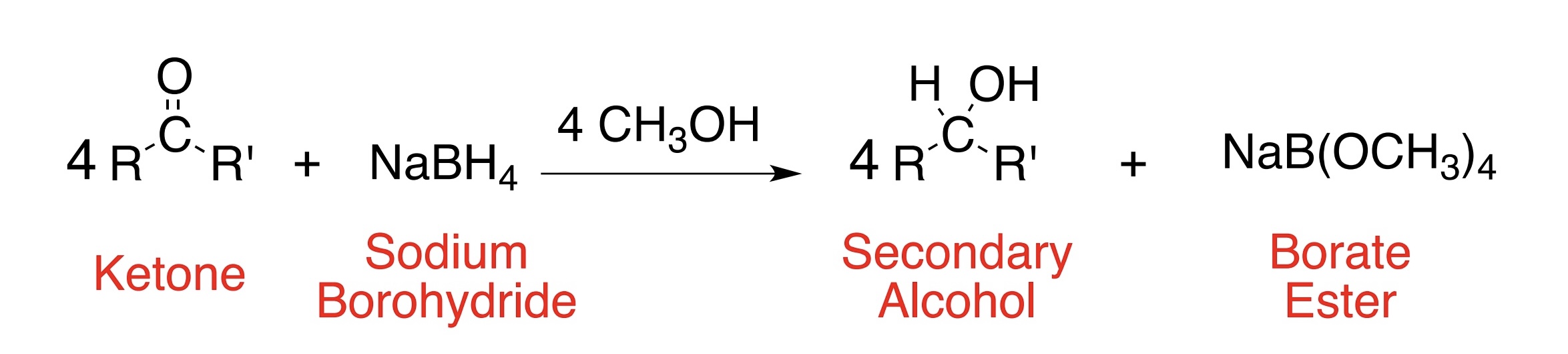
What is the product of reduction of a ketone using NaBH4?
Secondary alcohol
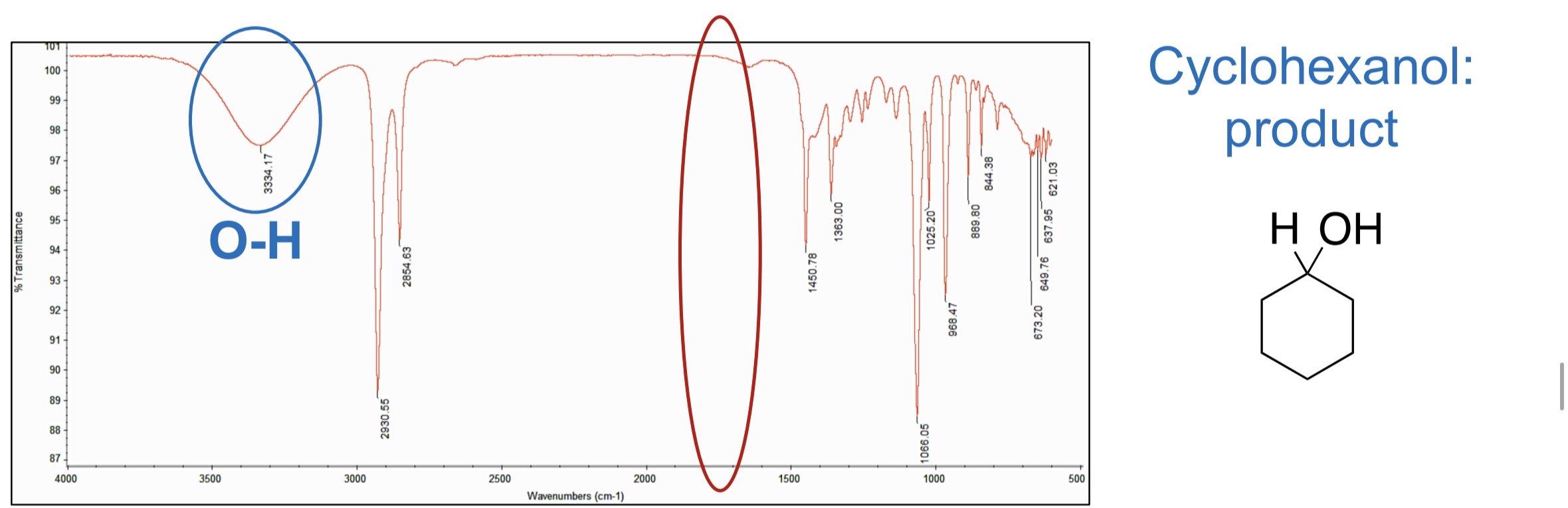
What peak is is now present?
O-H
What is the frequiency of sp2 C-H?
What is the purpose of adding HCl?
HCl neutralizes excess NaBH4
What is the IR frequency of sp3 C-H?
2850-3,000 cm-1
What is the IR frequency of O-H?
3,300-3,600 cm-1 (broad peak)
What is the IR frequency of C=O?
1650-1725 cm-1
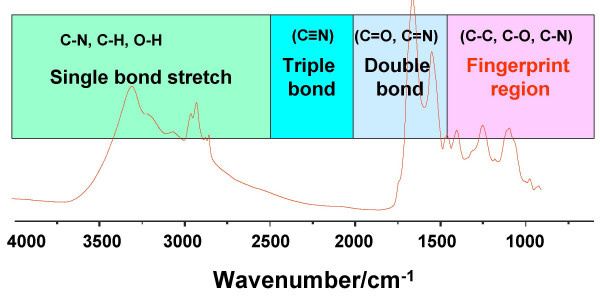
What is the reaction mechanism?
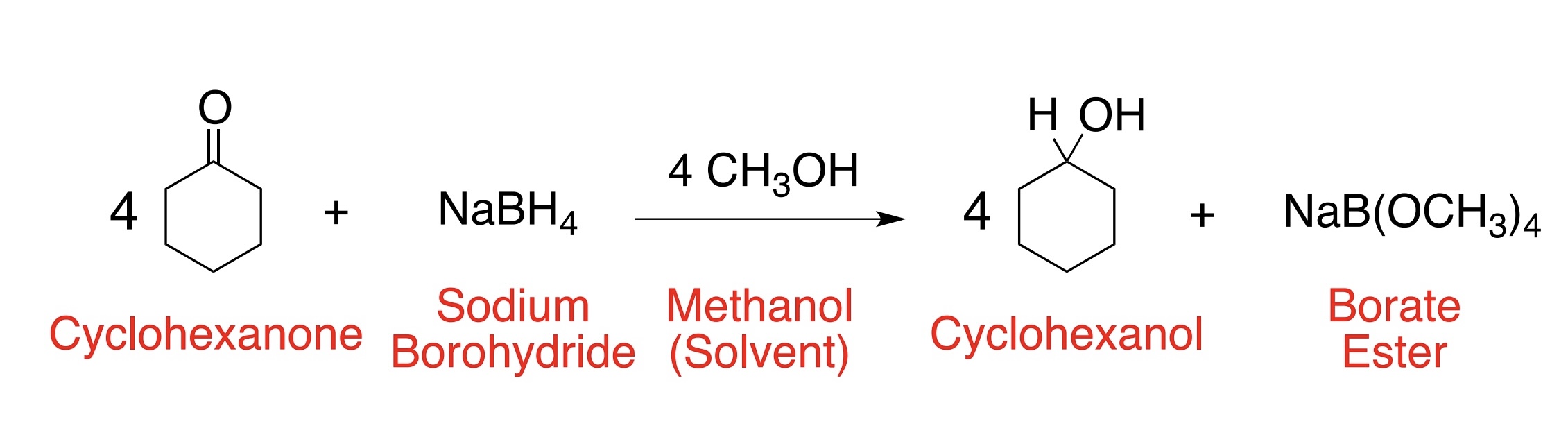
What is the purpose of sodium borohydride (NaBH4)?
NaBH4 is selective, mild reducing agent. Reduces ONLY aldehydes or ketones
One mole of sodium borohydride can provide 4 hydride ions (H:-) and therefore can reduce four moles of an aldehyde or a ketone
what are two different combinations of liquids that are immicible and can be used in a liquid-liquid extraction?
Ether (Top) and H2O
Methylene chloride and H2O (Top)
Name 4 compounds that are insoluble in water
Diethyl ether
Methylene Chloride (CH2Cl2)
Chloroform (CHCl3)
Carbon Tetrachloride (CCl4)