832 exam 2
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
architectural features common to all cells
plasma membrane-most basic compartmentalization
cytoplasm and nucleus-interior compartments
cytoplasmic organelles surrounded by lipid membrane
glycoproteins
oligosaccharides attached to proteins through asparagine or serine bonds
glycolipids
phospholipids w a saccharide attached
glycocalyx
makes surface of cell extremely hydrophilic, restricting passage of hydrophobic molecules through plasma membrane (carbohydrates are hydrophilic)
membrane purpose
serves a protective function, creates opportunity to regulate
how is cell compartmentalization achieved
mainly by lipid membranes
plasma membrane and most other organelle membranes are
lipid bilayers, regulates movement of molecules in and out of the cells/organelles
phospholipid
hydrophilic head group and hydrophobic tail, connected by glycerol backbone, restricts movement of polar hydrophilic molecules between in and out of the cell—> repelled by tail in middle
proteins in membrane
span, embedded in one side, or attached peripherally to membrane, regulatory
integral protein
part of the membrane, span or embedded in it(ions, receptors, transporters)
carbohydrates in membrane
form glycocalyx on exterior side of membrane(contains glycoproteins and glycolipids)
passive transport
main transport for small molecules and ions
simple diffusion-
high conc. to low conc.
move with the concentration gradient (rock downhill)
cross membrane freely(gases, H2O, steroids(hydrophobic/lipid soluble))
facilitative diffusion
MUST bind to specific transport protein
exhibits saturation kinetics—> will only happen at a certain rate
most drugs use this transport
pores, gated channels, carrier proteins(drugs use)
gated channels require stimulus to open(chemical or voltage)
change in confirmation to let ions through
active transport
main transport for small molecules and ions
REQUIRES ATP/electrochemical gradient
movement against concentration gradient
hydrolysis of ATP and phosphorylation causes conformation change in protein, which allows to go from low to high
electrochemical gradient can be used to generate ATP
also used to co-transport another molecule against its gradient
one mechanism by which drug resistance occurs
drug pumped out of cell before can bind to target
vesicular transport
macromolecules(lipids, proteins) and complexes, movement determined by process
endocytosis- invagination of plasma membrane, encloses them in vesicles and moves into cell
phagocytosis—> pathogens
receptor mediated endocytosis- receptor bound to ligands(insulin) and then engulfed in coated vesicle
exocytosis- vesicle inside cell fuses with membrane and content dumped into the extracellular fluid—> vesicles come from golgi and lysosomes
contain molecules/proteins or cellular waster
transport cells use
cells use a combination of all of these within the cells for them to do their job, work together to maintain cellular homeostasis
lysosomes
digestive organelles, breakdown a variety macromolecules
surrounded by lipid bilayer, lumen contains acid hydrolases(only function at low pH)
5.5 pH maintained by ATPases that pump H+ against gradient into lumen
products broken down are recycled by anabolic processes or excreted as waste
can fuse w other vesicles(ex. phagosomes fuse w lysosome and contents are degrades/injured or dead cell)
Autophagy- forms auto phagosome to breakdown damaged organelles/debris from cytoplasm
mitochondria
energy generators of the cell, produce majority of ATP
has inner and outer membrane (inner membrane is folded several times, highly impermeable)
almost everything inside comes from transporters
houses mitochondrial matrix
cristae holds ETC and ATPsynthase
enzymes are involved in fuel oxidation(TCA cycle and beta oxidation)
also found within matrix, DNA that encodes several ETC proteins
golgi complex
membranous organelle involved in sorting and distribution of proteins and lipids synthesized in the ER
vesicles containing lipids or proteins from ER fuse with golgi
the sorted and packaged into different vesicles w different fates and functions
lysosomes and secretory vesicles produced in golgi
proteins post translational modified here, especially with sugars!
endoplasmic recticulum
network of membranous tubules with multiple essential cellular functions
smooth ER-
contain enzymes for synthesis of lipids
site of drug and toxin metabolism by P450 enzymes
storage of glycogen
rough ER- in muscle cells its sarcoplasmic recticulum
ribosomes located on outer surface
major sites of protein synthesis from mRNA
site of initial storage of newly synthesized proteins(determine where go)
site of some post translational modifications—> N linked glycosylation
cellular cytoskeleton
organizes structure and shape of cell as well as arrangement of sub-cellular organelles and movement of vesicles
mictotubules
cylindrical tubules made of tubulin subunits
position organelles in cytoplasm
critical for movement of cellular vesicle
form mitotic spindle in cell division
microtubule formation is target of several cancer drugs
actin filament
made up of actin subunits
control cell shape and movement
cell division, contraction, phagocytosis, any movement involved in normal organ function
intermediate filaments
different types made of distinct fibrous proteins
lamin, vimentins, keratin
provide structural support for plasma and nuclear membrane
provide stability to cells under conditions of mechanical force
nucleus
houses genetic material in cell and largest of subcellular organelles
double lipid bilayer membrane(nuclear envelope) w special pore structures for entry and exit of proteins and RNA
ribosomes are found on outer membrane since continuous with rough ER
nucleolus- does not have membrane, consists of an aggregation of genes encoding ribosomal RNA and proteins that regulate them
genetic material exists in nucleus as complex of DNA and proteins called chromatin
heterochromation: tightly compact
euchromatin: loosely compact—> location of transcribed genes
DNA divided into chromosomes besides germ cells, human cells—> 46 chromosomes
2 copies each of 22 + 2 for sex
site of DNA replication and repair as well as transcription (RNA synthesis) and splicing of RNA into mRNA
chemical messangers
carry out intercellular communication that elicits cellular responses through the process of signal transduction—> many drugs alter these pathways that are misregulated in disease
key concepts in cell signaling
a stimulus(change in cell environment) triggers a cell to secrete a chemical messenger
this messenger then acts on target cell by binding to specific receptor
this biding triggers a cascade of events that elicits a response—>signal transduction
often transmitted to nucleus to cause changes in gene expression
finally the signal and response are terminated
chemical messangers
often small molecules or proteins(acetylcholine, insulin)
Endocrine(hormones) Messaging
secretory cell and target cells are distant, chemical messenger must travel through the blood, often many target tissues(insulin)
Paracrine Messaging
secretory cells and target cells in close proximity, often messengers secreted by exocytosis(cytokines,acetylcholine)
Autocrine Messaging
the secretory cell is the target cell(t lymphocyte cells)
Juxtacrine Messaging
involves direct contact between cells(touching), one with ligand and the other with receptor, ligand not secreted(t cells and dendritic cells)
ligand binding
the binding of ligand to its receptor triggers a conformational change(change in shape) in the receptor that alters its function
intracellular receptors
located inside the cytoplasm or nucleus(ligands are small hydrophobic molecules readily able to cross the membrane)
plasma membrane receptors
located at cell surface and usually are transmembrane proteins(hydrophilic signaling molecules that cannot cross the membrane)
steroids/intracellular signal transduction
hydrophobic ligands that can enter cell by simple diffusion, are planar so can slide through. signal is transduced from outside the cell into the nucleus by the steroid bound to its specific receptor
receptors are examples of ligand-activated transcription factors
the change in shape uncovers a domain that binds specific DNA and allows dimerization of 2 identical receptors—> two receptors bind to each other
receptors enter nucleus, binds to genes and alters the amount of transcription
plasma membrane receptor transduction
confirmation change “activates” the intracellular domain of the protein
this can be an opening of a channel(gated channels), activation of enzymatic activity(kinases often phosphorylate themselves other kinase nearby—> MAP) or change in activity of proteins bound to the receptor(final receptor is not a kinase—> GCPR)
signal is transduced to the nucleus by other proteins
proteins bound by a second messenger alter gene expression through regulation of transcription factors
signaling pathways
regulate transcription factors to alter gene expression and elicit a cellular response
specific for each transcription factor and depends on the amino acid sequence of the DNA binding domain
the transcription of these genes will be regulated by the transcription factor and signaling pathway that targets it
activators
increase the rate of transcription
repressor
decrease rate of transcription, turning off pathway
how signal transduction terminated
chemical messenger ceases to be released or is degraded
receptor levels decrease due to internalization and or degradation
phosphatases remove activating phosphorylation from receptors
second messengers are degraded
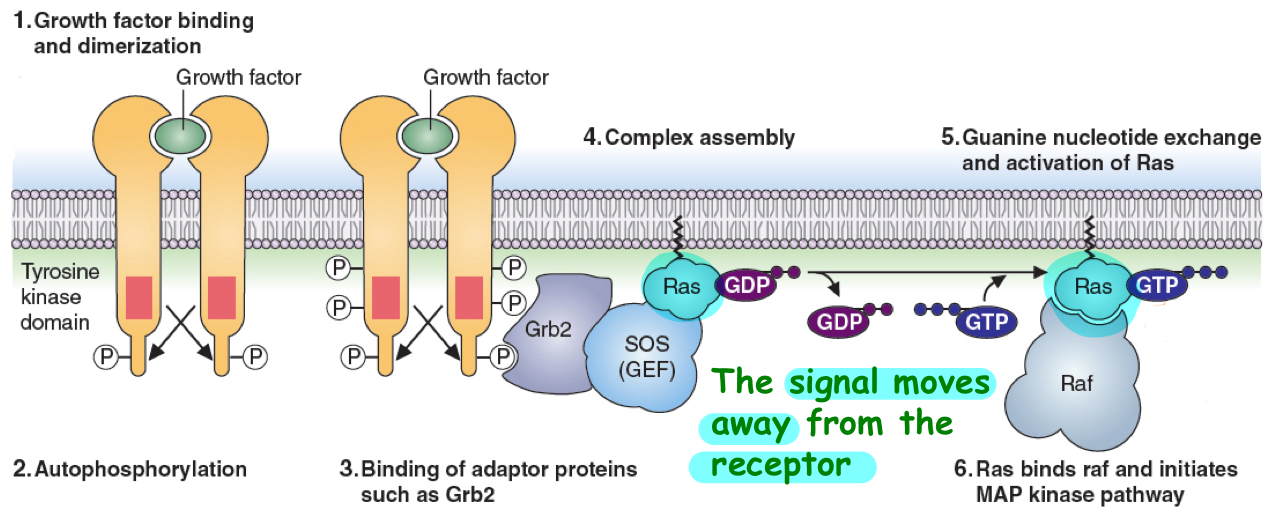
tyrosine kinase receptors
plasma membrane receptor example
RTK is activated upon ligand binding and phosphorylates itself on a certain tyrosine residue(autophosporylation)
and/or to phosphorylate other associate proteins
this phosphorylation of these amino acid residues turns them into binding sites for other proteins which in turn transduce the signal into the cell
MAP kinase example of this- kinase cascade
binding event activates proteins in these complexes which often have enzymatic activities
Raf—> kinase which is activated by Ras
Ras—> signal transducer protein
signal moves away from receptor into the cell
may be multiple signal transducer proteins—> keep phosphorylating each other down the line til reach intracellular target
persistent MAPK signaling causes unregulated cell proliferation
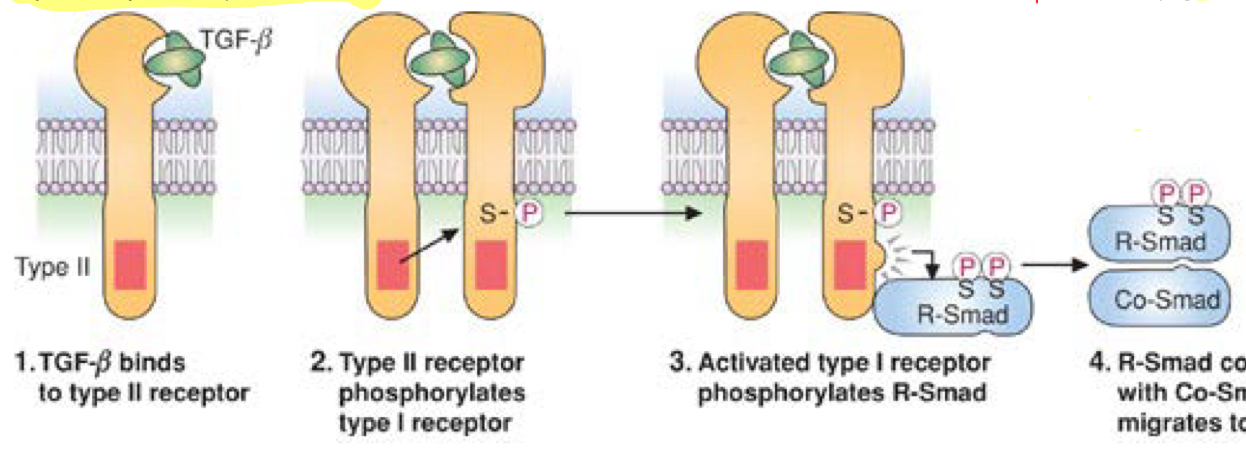
serine/threonine kinase
plasma membrane receptor example
TGF-beta signaling- assembly of transcription factor
cytokine alters the receptor so that it can bind to a second receptor and phosphorylate it at a serine/threonine residue—> heterodimer
second receptor then bind to a protein and phosphorylated it(R-Smad)
this complex goes to nucleus where it binds genes and changes transcription
R-Smad must be phosphorylated to then interact with co-Smad protein to actually then travel to nucleus
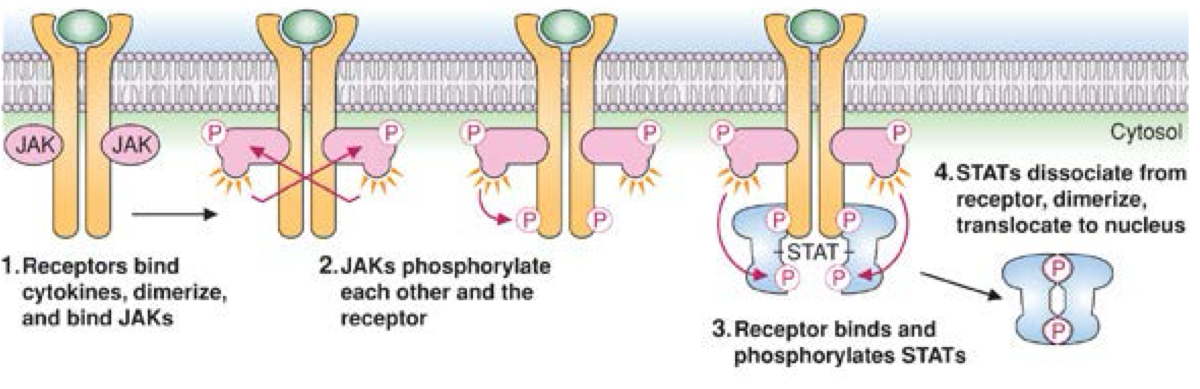
Jak-Stat signaling
binding of cytokine(paracrine) activates receptor to dimerize
binds Jak proteins which then phosphorylate each other and the receptor
STAT proteins bind and then phosphorylated by Jak Proteins
this triggers stats to dissosiate from receptor and dimerize
these move to nucleus to bind to genes, alter transcription
assembly of transcription factor
deals with inflammatory pathways
THESE RECEPTORS ARE NOT KINASES
GCPRs (heptahelical or g-coupled protein receptors)
G proteins are tethered to the membrane by lipid anchors
binding of ligand to the receptor triggers cellular response through the change in production of a second messenger
in absence of ligand they associate w heterotrimetic(3 subunit alpha, beta,gamma) g-proteins
second messenger is a small diffusible signaling molecule—> production is altered in response to a stimuli
they bind to effector proteins within cell to exert a cellular response—> different alpha subunits regulate production of different secondary messengers
Galpha(s)- increases cAMP production
Galpha(i/o)- inhibits cAMP production
Galpha(q/11)- increases DAG, IP3 and calcium
the complex breaks apart and away from receptor upon binding of ligand
alpha subunit exchanges GDP for GTP
the GTP bound to Galpha(s) binds to adenylate cyclase and activates it to make cAMP
eventually Galpha(s) hydrolyzes the bound GTP to GDP, inactivates itself, and reassociates with receptor and other subunits
once second messenger is produced (cAMP in this case) it diffuses through cytoplasm and binds to specific proteins
Example exam questions:
1. Signal transduction is the process by which:
a. chemical messengers find their target cells
b. second messengers are degraded
c. chemical messengers are secreted by cells
d. signals are transmitted from the plasma membrane to the nucleus
e. receptors are internalized in vesicles
2. Which of the following is TRUE about paracrine horomones?
a. they are secreted by one cell and act on a nearby cell
b. they act on the same cell from which they are secreted
c. they travel through the blood to reach target cells
d. they do not bind receptors
e. none of the above
3. Cytokines….
a. are lipids attached to proteins
b. are chemical messengers that work by the paracrine mechanism
c. bind intracellular receptors
d. are transcription factors
4. MAP kinase signaling……
a. is initiated by activation of a tyrosine kinase receptor
b. involves a kinase cascade
c. is sometimes hyperactivated in cancer cells
d. can induce cells to divide
e. all of the above
d
a
b
e
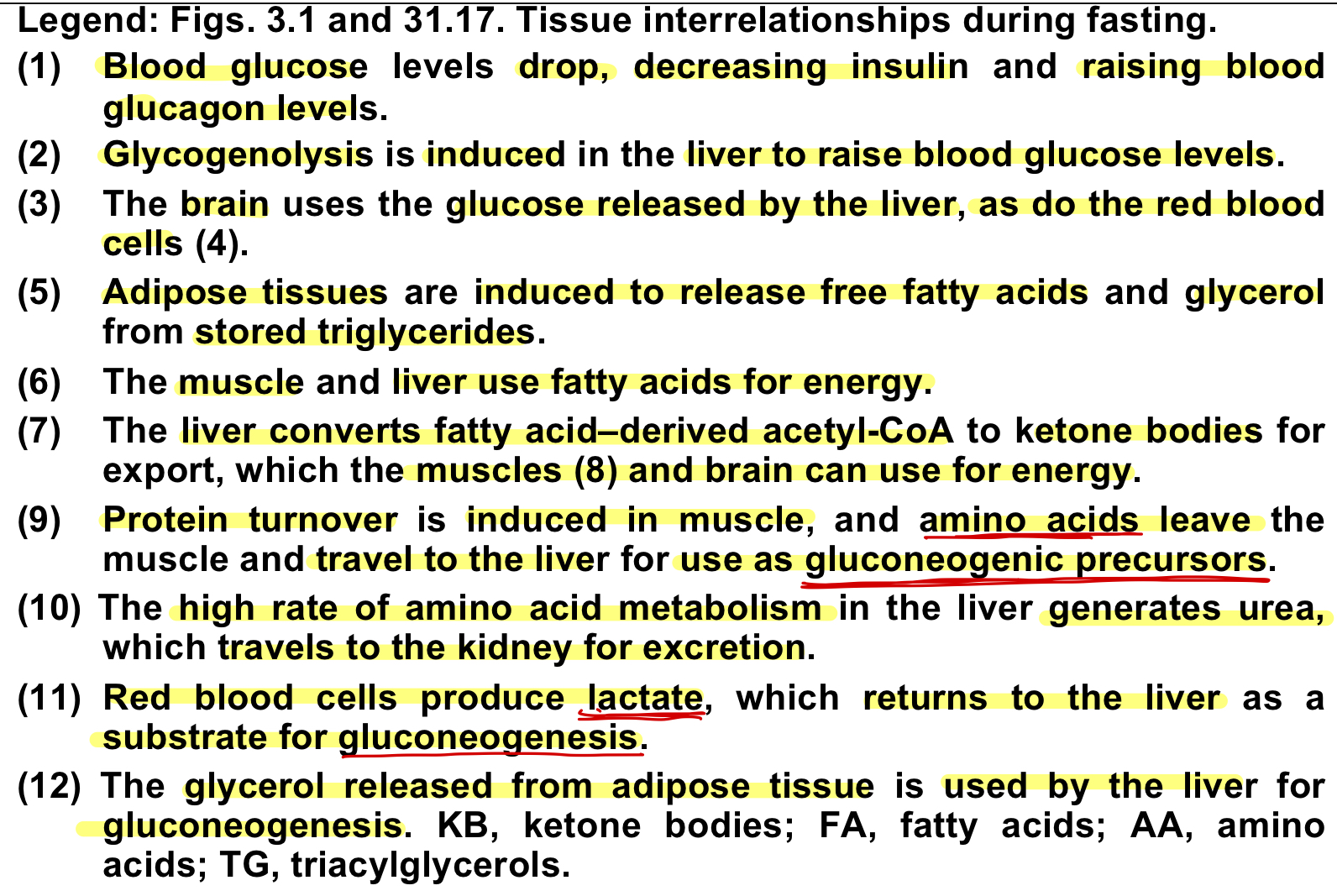
common biological energy utilizations(uses energy)
biosynthesis
detoxification
muscle contraction
active ion transport—>ATPase
thermogenesis
common biological energy production via oxidation of:
carbohydrates(sugars)
lipid
protein
O2 does not turn to CO2, reduced to H2O in mitochondria during the ETC
CO2 is from fuel decarboxylate
energy content of simple fuel molecules
carbohydrate: 4
fat: 9—> least oxidized
protein: 4
alcohol/ethanol: 7
what regulates the fed state
insulin, lowers blood sugars—> dictates movement of sugar from blood into the cells
sugar is either stored(glycogen) or metabolized to fatty acids—> shuffle from one to the other
can build fat from sugars
lipogenesis—>acetyl-CoA converted to fats
high caloric intake turns into storage
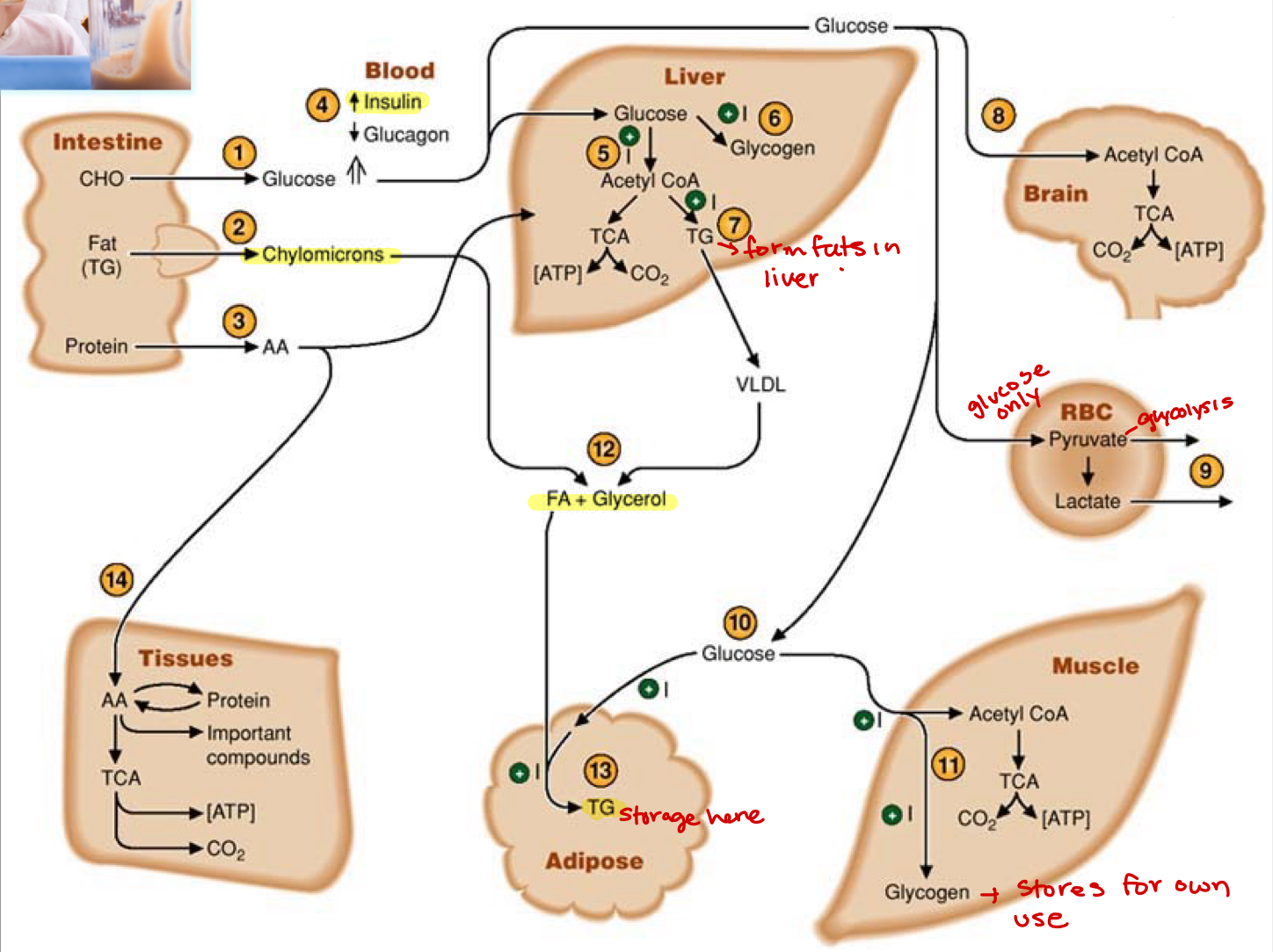
glucagon(fasting state)
glycolysis is triggered by rise in glucagon
fat=triglyceride(fat storage)=esters
triglyceride- 3 ester groups
ester-fatty acid+alcohol w water elimination/condensation
breaks down triglycerides through hydrolysis, opposite is condensation rxn
lipids
includes:
triacylglycerides(fat and nonpolar)
phospholipids(phosphate esters, glycerol backbone and charged)
phosphatidylcholine=lecithin
Steroids(cyclic and lipophilic)
cholesterol—> waxy fat-like substance
fatty acids
fatty acids
triglyceride is 3 fatty acids combined
saturated—> bent tail
unsaturated—> unbent
cis or trans describe the double bonds
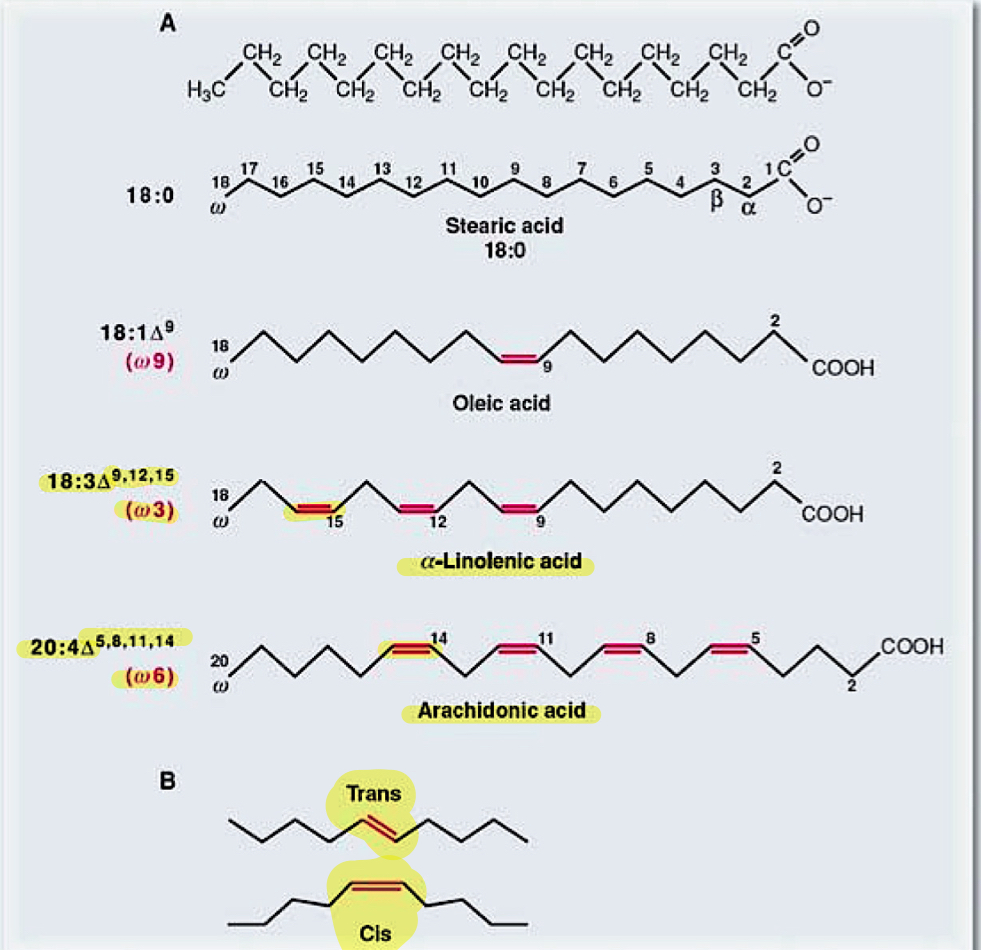
w, triangle symbol (FA chains)
first position of double bond in relativity to omega
#of double bonds
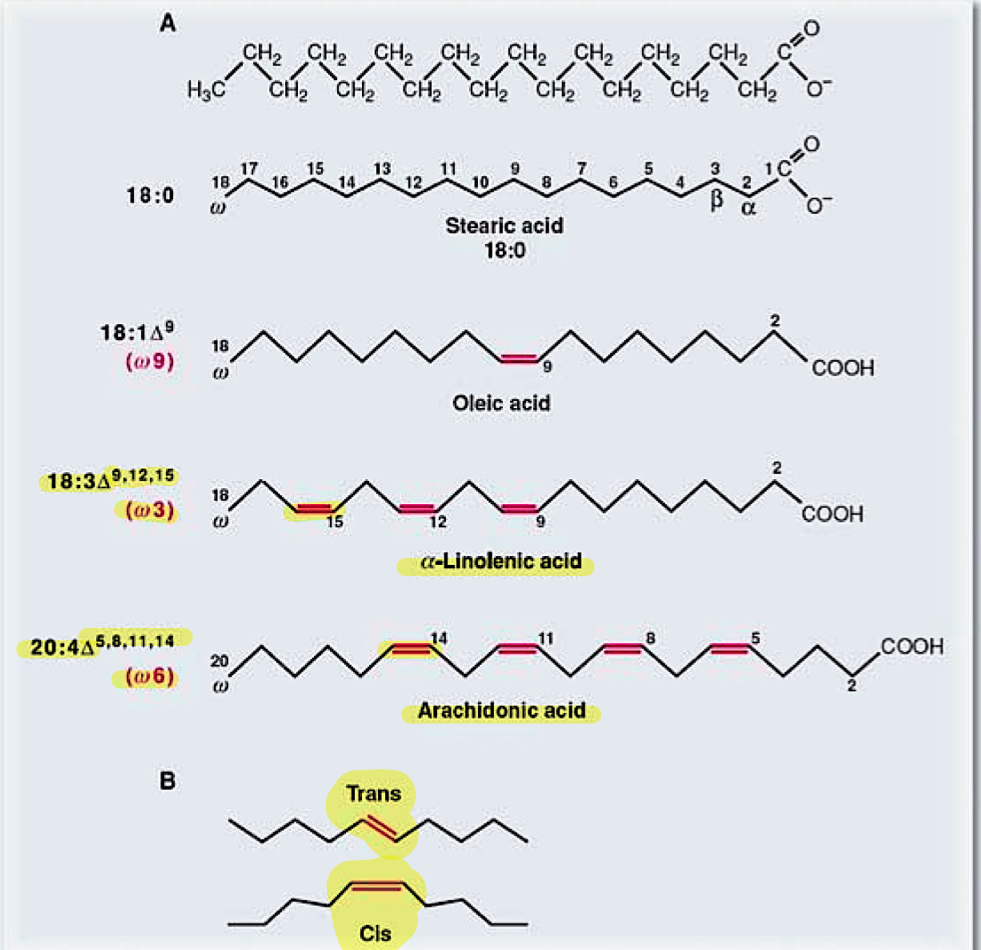
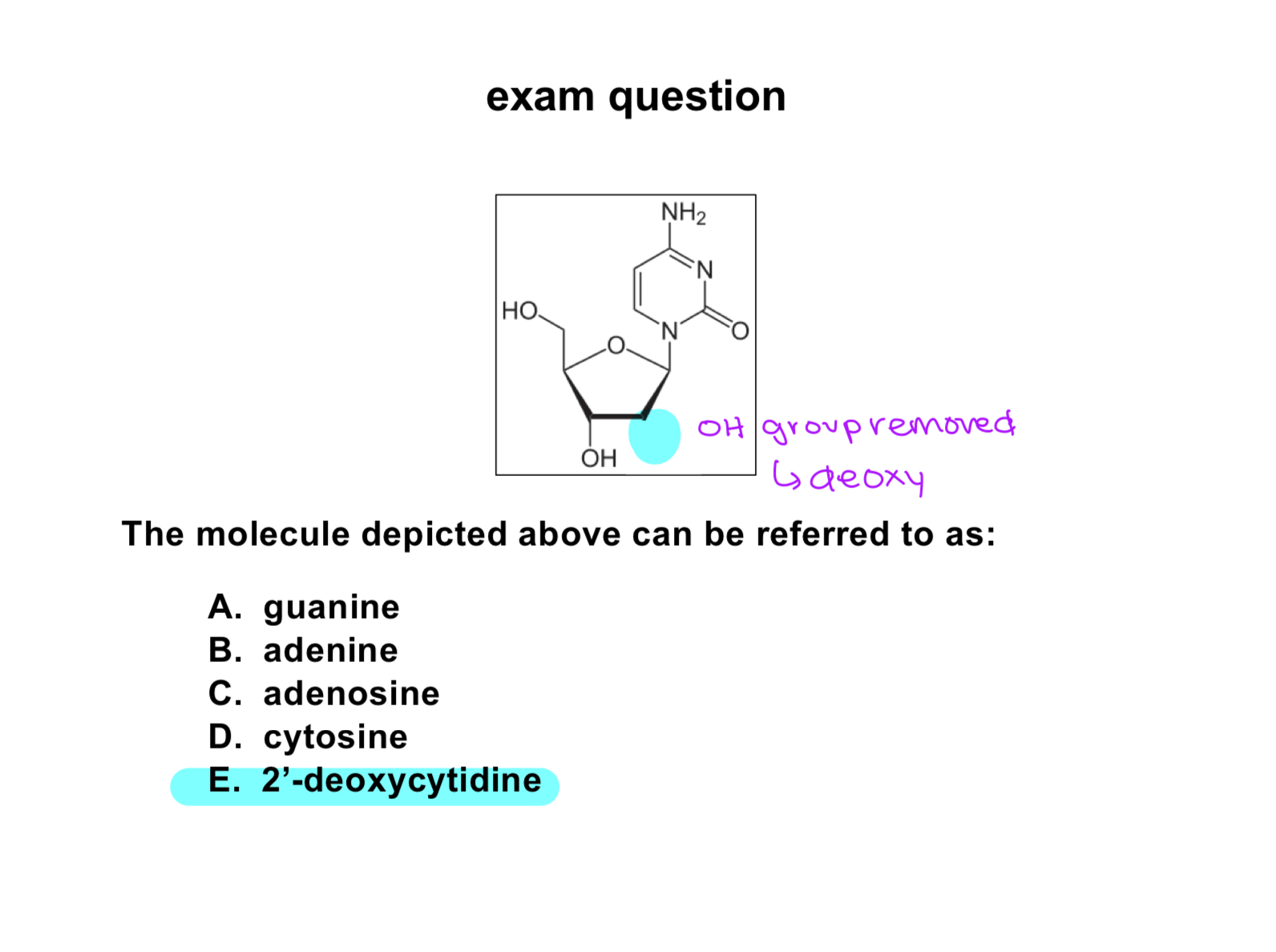
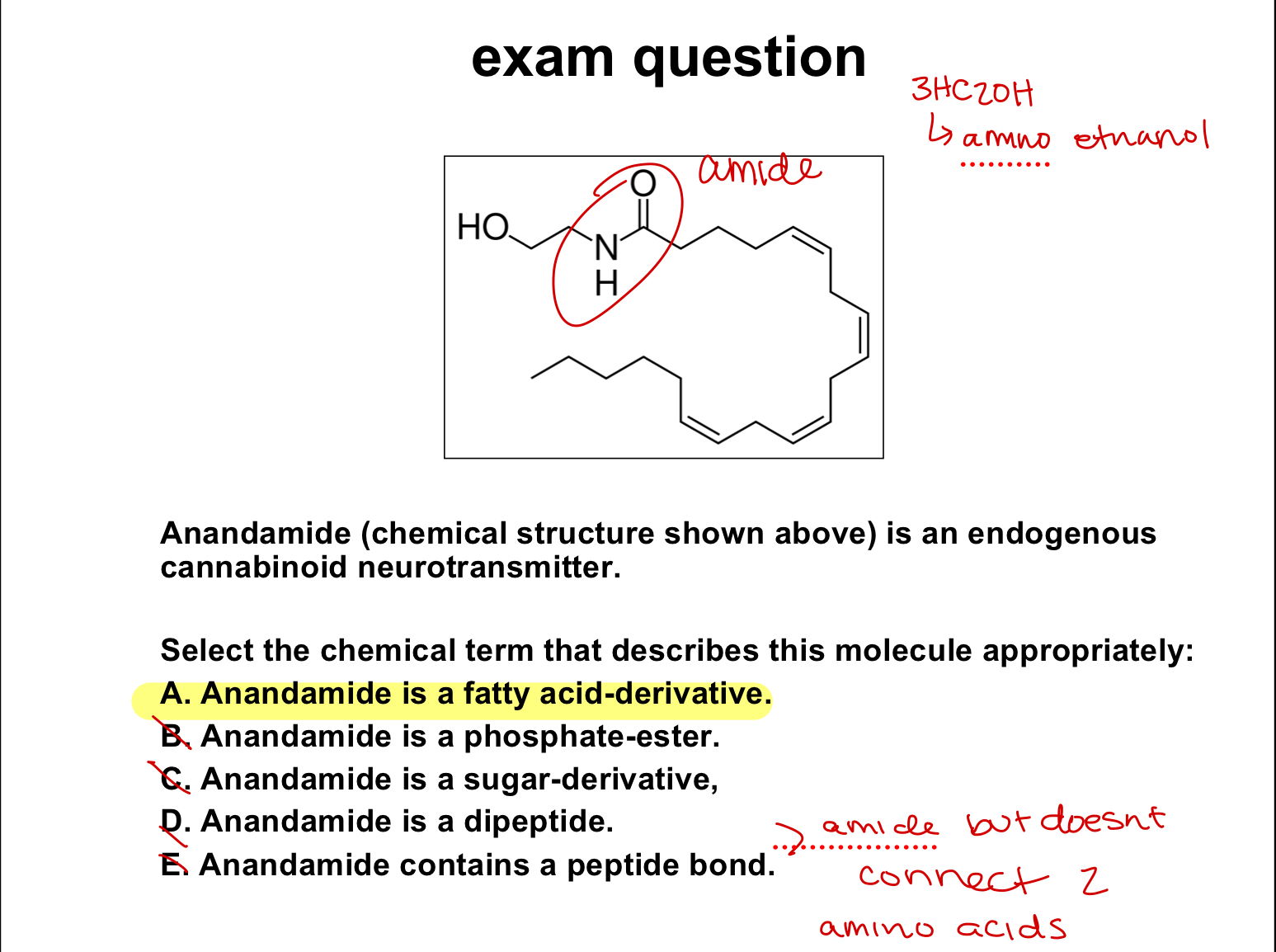
exam question:
select the chemical term that describes this molecule appropriately:
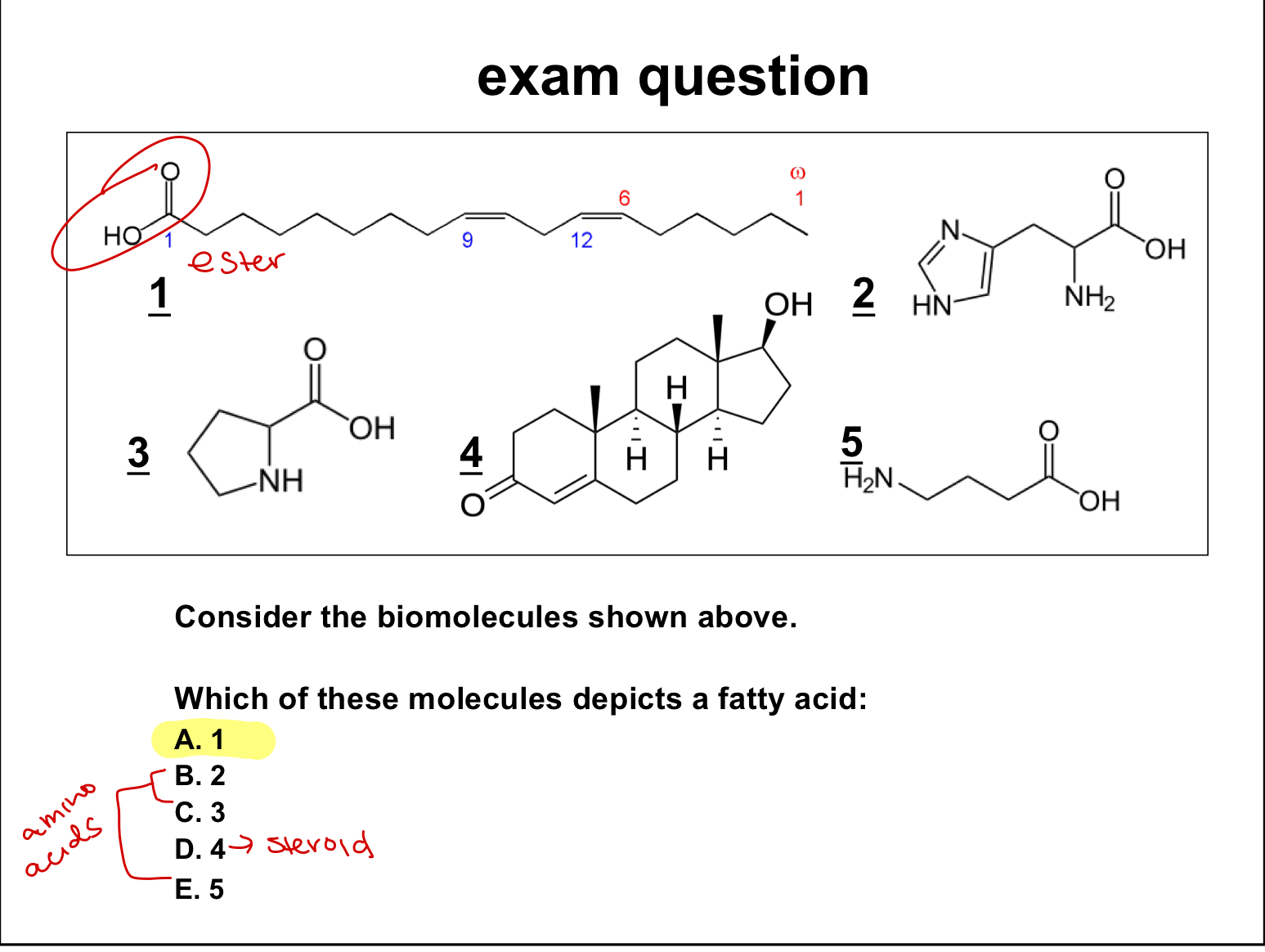
exam quesiton:
which of these molecules depicts a fatty acid?
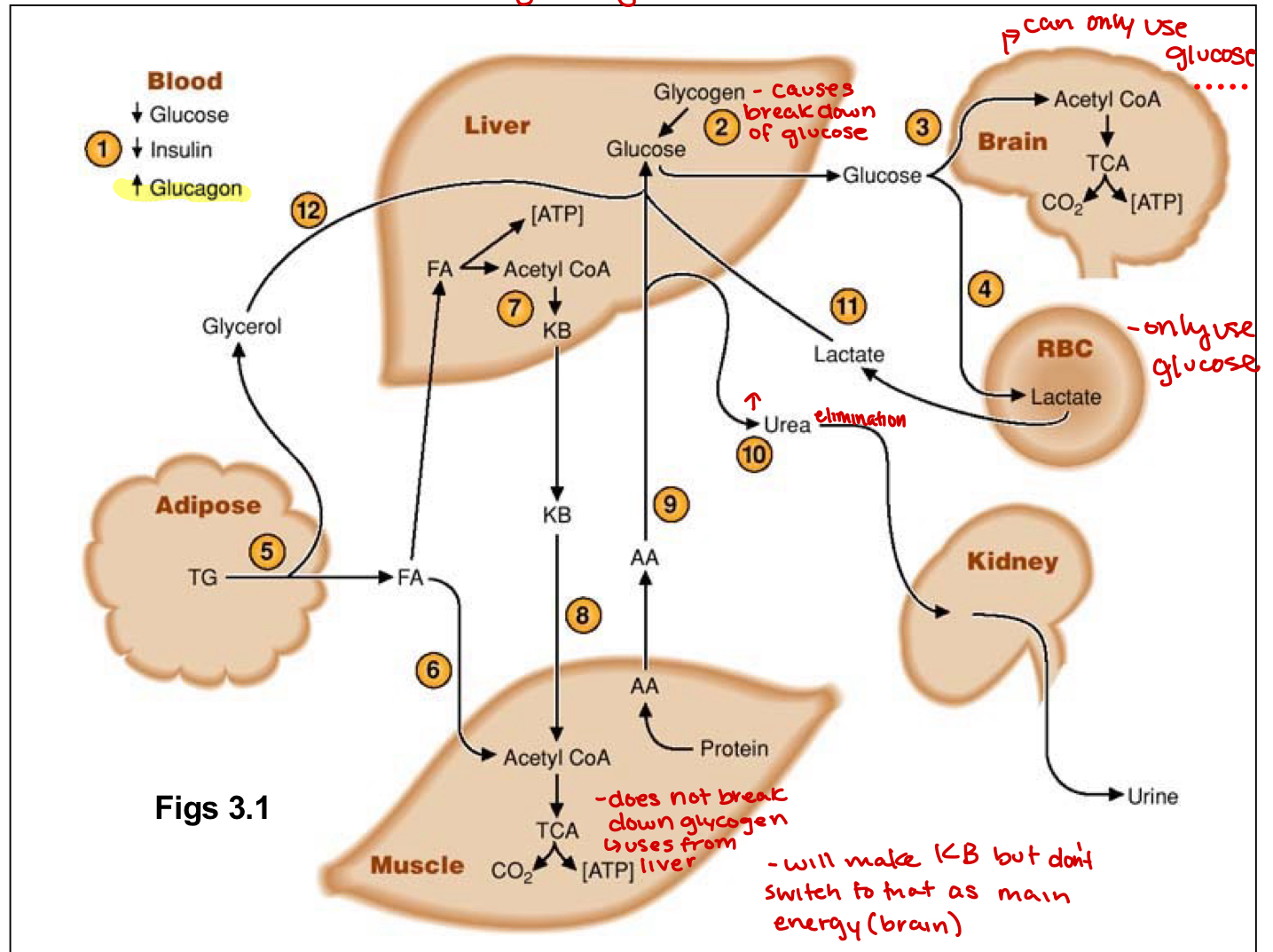
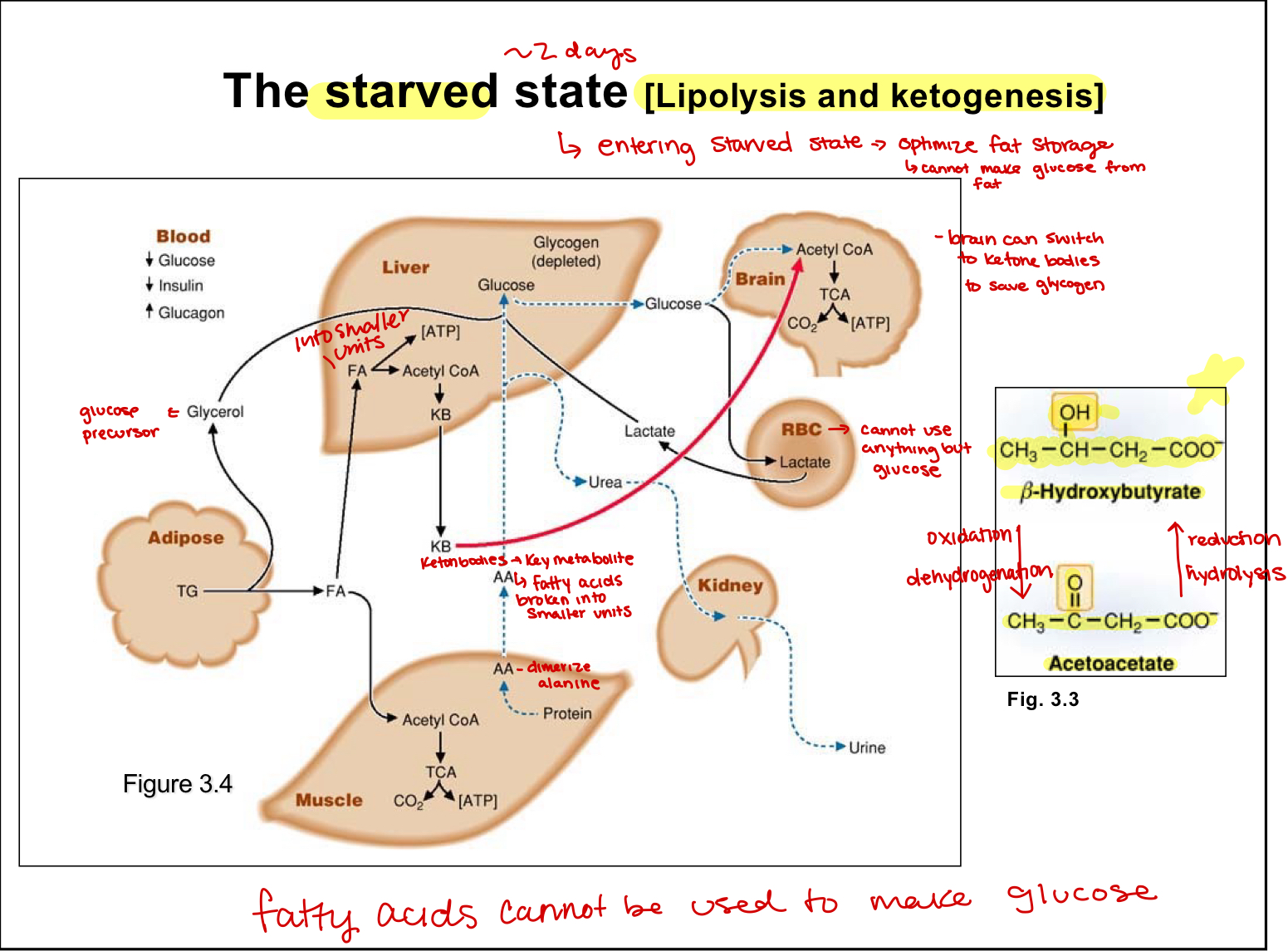
ketone bodies
formed in the liver during ketogenisis, liver switches to produce this for muscles and brain during starved state
ketoacidosis
pathological metabolic state marked by extreme and uncontrolled ketosis(high ketone conc.)
glycogen synthesis
after meals, insulin acts on hepatocytes to stimulate action of enzymes such as glycogen synthase
glucose transformed into glycogen as long as insulin and glucose remain plentiful
Fed state- liver takes up more glucose than released
this is an energy consuming process
glycogenolysis
when there is a need for energy, glycogen is broken down and converted back into glucose
glycogen phosphorylase primary enzyme for glycogen breakdown
enzymatic conversion of glycogen polymers to glucose monomer
takes place in liver and muscle tissues
liver can consume glucose6phosphate or remove phosphate group to release free glucose molecule into blood for other cell use
muscles will keep glucose they make as they cannot remove the phosphate
during fasting state
exam question:
which of the following biomolecules does not serve as fuel for energy production?
a. amino acids
b. fatty acids
c. ATP
d. triglycerides
e. glucose
c
leptin
protein hormone that plays key role in regulating energy intake and energy expenditure
includes appetite and metabolism
most important adipose derived hormone—> made in fat tissue
chylomicrons
lipoproteins produced in gut(intestinal epithelial cells) after fat digestion and secreted into lymph then makes to blood—>carries fat through blood(since not water soluble)
exam question:
select the correct term that describes the transport form of dietary fat in the blood stream after systemic absorption through the intestinal epithelium?
a. trigylceride
b. insulin
c. VLDL
d. chylomicrons
e. glycogen
d
exam question:
select the correct statement that describes a metabolic change that occurs in the fasting state:
a. hepatic glycogenolysis generates glucose that enters the bloodstream
b. elevation of serum insulin levels induces glycogenolysis
c. ketone bodies are used for gluconeogenesis
d. triglycerides serve as fuel for RBC
e. glucose released by muscle cells is used for brain energy metabolism
a
exam question:
amino acids can serve as substrates for gluconeogenesis. Select the correct molecule that serves the purpose of urinary nitrogen excretion:
a. ammonia
b. glycine
c. formaldehyde
d. urea
e. creatine
d
gluconeogenesis(starved state)
protein turnover induced in muscles and amino acids travel to liver to use as precursors
lactate produced by RBC returns to liver to be used as a substrate
ketogenesis here, can make out of FA from the adipose tissue
glycerol released from adipose tissue then used by liver
lipolysis occurs in adipose tissues and is glucagon driven
stores and mobilizes when needed
glycosidic bond
sugar- sugar bond, see in glycogen production—> takes energy to produce
oxidoreductases
catalyze oxidation-reduction rxn
ex. dehydrogenases
transferases
catalyze C,N or P group transfer rxn
ex. kinases
hydrolases
catalyze cleavage of bonds by water(hydrolytic) rxn
ex. proteases—> HIV and viral disease
isomerases
catalyze racemization/isomerization rxn—> rearrange/reorganize parts of molecules=isomer
ex. triose phosphate isomerase
isozymes
enzymes that differ in amino acid sequence but catalyze the same rxn
they will have different Km s that help regulate/fine tuning of metabolism
ex. hexokinase(low Km) and glucokinase(high Km) with glucose storage
enzyme action as biocatalyst
does not change the delta G
lowers the energy barrier of the reaction to make it more favorable
catalytic enzyme will not be consumed in the rxn
substrate
what is transformed in enzymatic rxn
cofactor
other ions that assist in enzymatic rxn
Zn in angiotensin conversion(ACE)
coenzyme
cofactors that take part in the transformation(participate in catalysis by rpoviding functional groups , often derived from vitamins
ex. NAD+—> vitamin derivative
active site
where substrate binds and transformation occurs on a enzyme
transition state
the energy barrier of a reaction
this is what is effected by enzymes
Km value
describes the affinity of the enzyme for the substrate
high Km value—> low affinity—> need more to efficiently bind
low Km value—> high affinity—> need less to efficiently bind
competitive inhibition
binding of active site of enzyme is competed for against substrate and inhibitor(drug)
mimics substrate—> substrate analog
if increase amount of substrate can out compete the inhibitor
increase the Km, does not change Vmax
inhibitors can stay bound to active site for a while or outcompete the substrate
inhibitor can have a higher affinity to enzyme than substrate
ex. ACE inhibitors, block action of enzyme by not allowing it to convert Angt 1 to angt 2—> binds better than the substrate itself
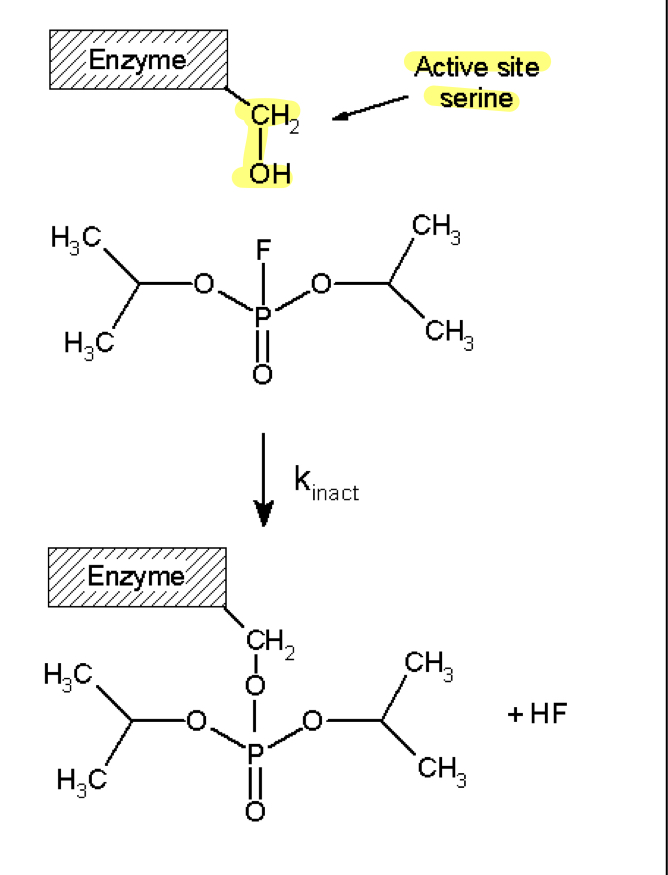
irreversible(covalent) inhibition
organophosphate group adducts with active site forming a covalent bond
this cannot be reversed and causes enzyme to be inactivated
adduction of enzyme—> suicide of enzyme
penicillin is good drug example of this—> adducts the enzyme
enzyme cascade
highly regulated—> all need to be activated and giving each other feedback whole time
ex. blood coagulation
(clotting)
allosteric modulation
inhibitor binds at a non active site(allosteric) which then does not allow enzyme to change confirmation to do enzymatic activity
inhibitor does not mimic the substrate
reversible, cannot be outcompeted by increase in substrate since it is non competitive
ex. non-nucleoside reverse transcriptase inhibitors(NNRTIs)
allosteric activation(effectors)—> enhance proteins/enzymes activity, decrease Km
allosteric inhibition—> decrease activity, increase Km
enzyme regulation—> modulators at allosteric sites tune enzyme activity to determine how active things are
use of ATP
mechanical work- actin/myosin movement in muscles relies on ATP
hydrolysis(water molecule attacks gamma P in ATP) of ATP by myosin head(ATPase)—> breakage of high energy bond allows mechanical movement
ion gradients
enzymes use ATP as cofactor to help push things against concentration gradient
biosynthesis(glycogen ex.)
ATP is cofactor for hexokinase in turning glucose into glucose 6-P
delta G, gibbs free energy
energy released or used up during a reaction, tells you how favorable a reaction is, can couple these together to make unfavorable rxn more favorable, use of ATP as cofactor example of this
enzymes DO NOT change delta G, make the transition state different by lowering energy barriers
ATP hydrolysis- 2 high energy bonds—> delta g of ATP is -7.3 for breaking one of these bonds
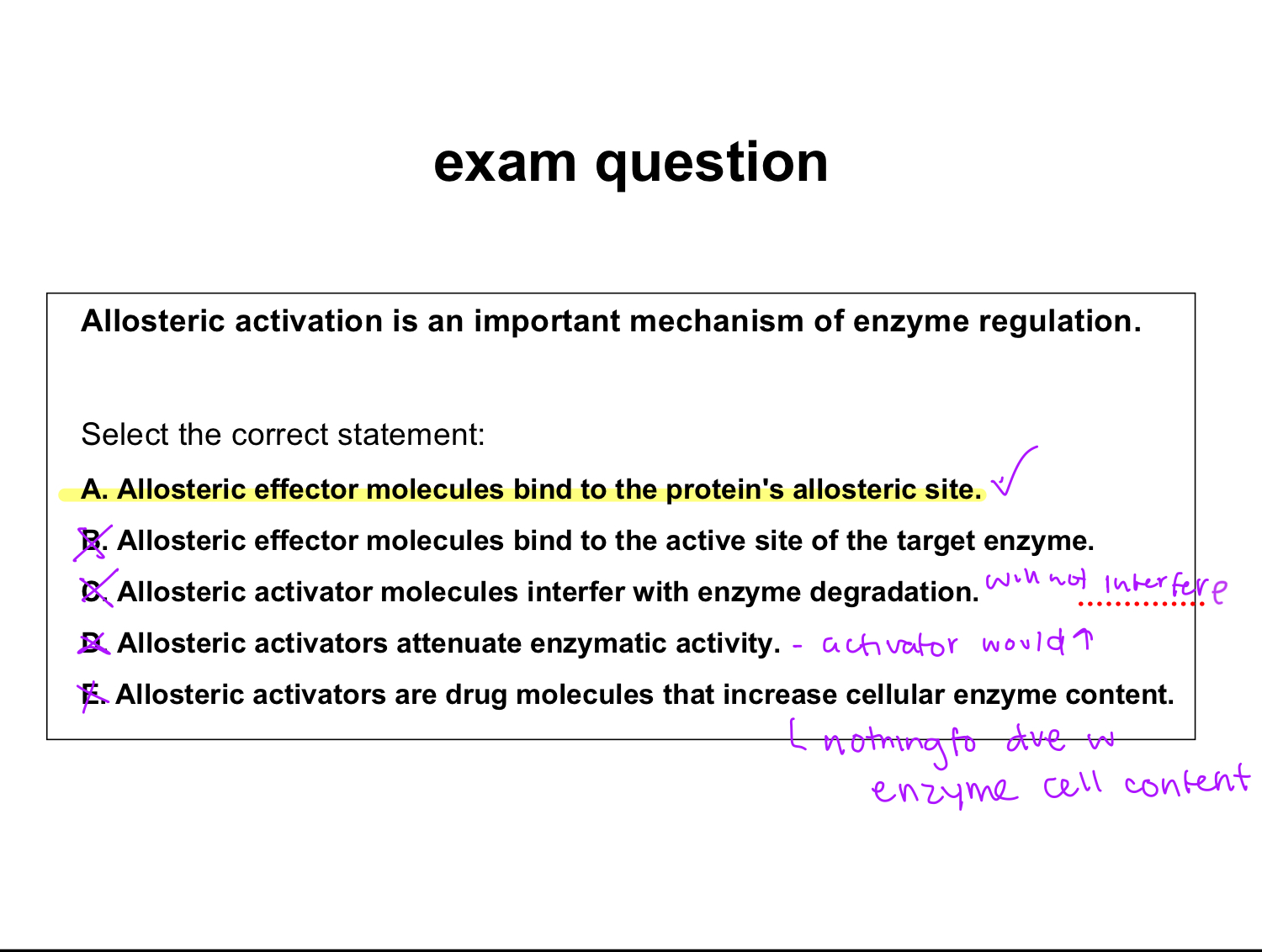
exam question:
allosteric activation is an import enzyme regulation. select correct statement:
a. allosteric effector molecules bind to the proteins allosteric site
b. allosteric effector molecules bind to the active site of the target enzyme
c.allosteric activator molecules interfere with enzyme degradation
d. allosteric activators attenuate enzymatic activity
e. allosteric activators are drug molecules that increase cellular enzyme content
a
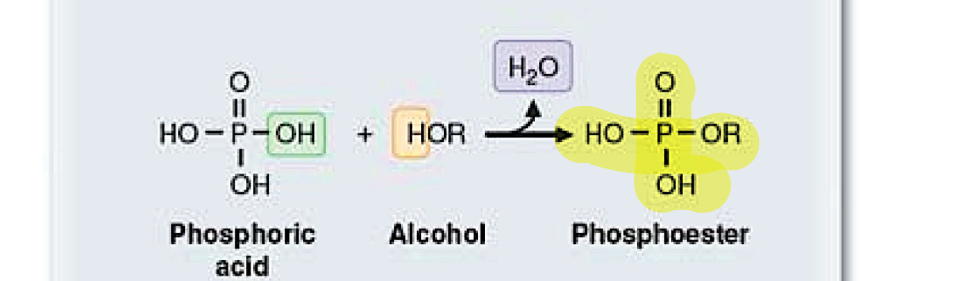
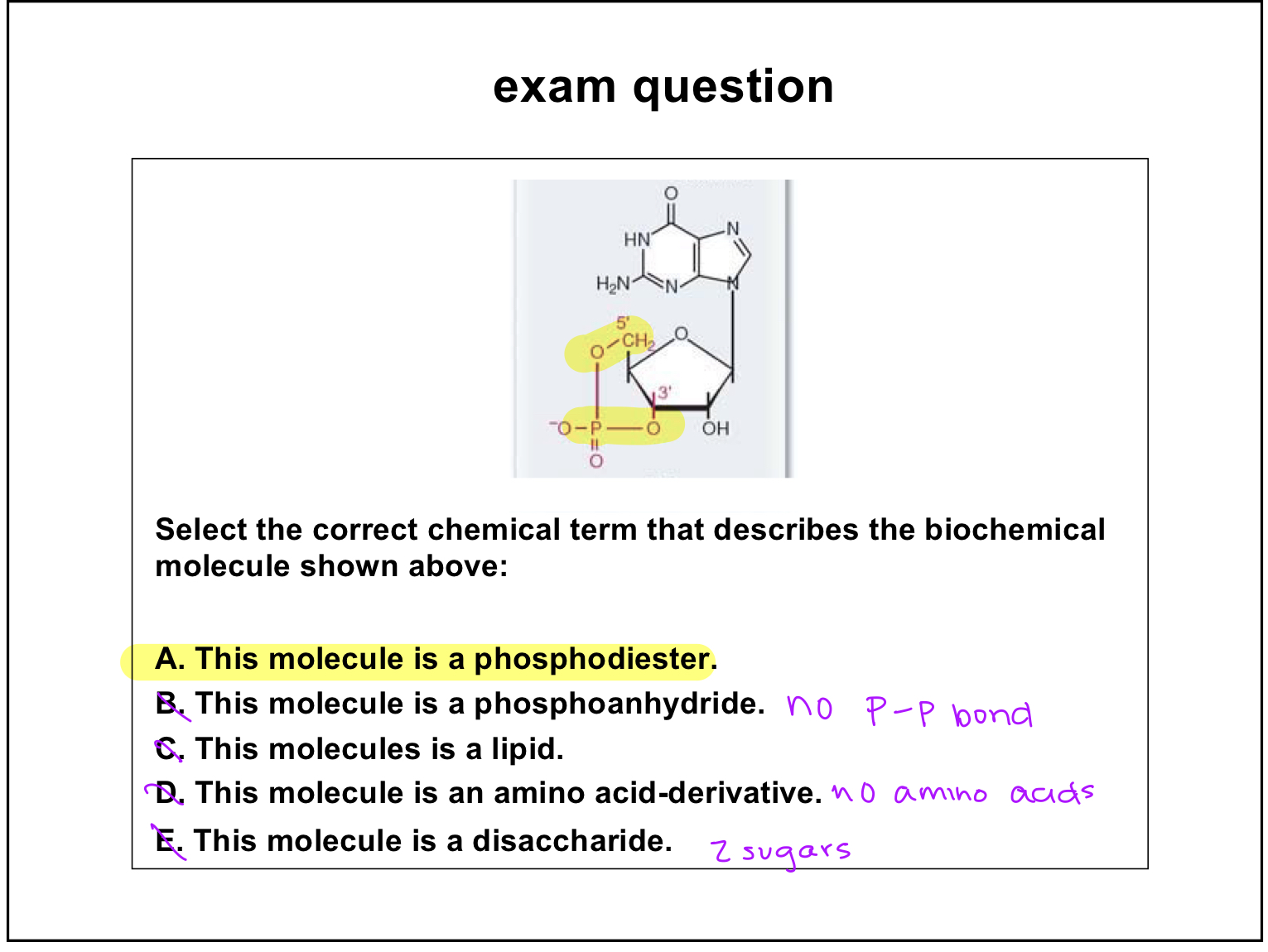
phosphoester
phosphoric acid+alcohol
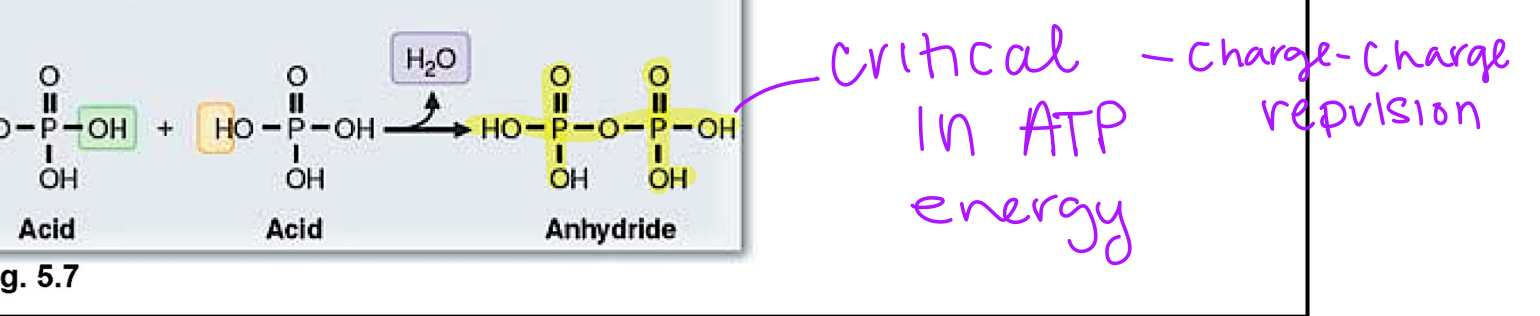
anhydride
acid+acid—> double phosphoric acid bond
cAMP metabolizing enzyme
cAMP phosphodiesterase—> hydrolysis to break
exam question
exam question
ATP energy content
-7.3 kcal/mol= delta G
this is release to drive future rxns
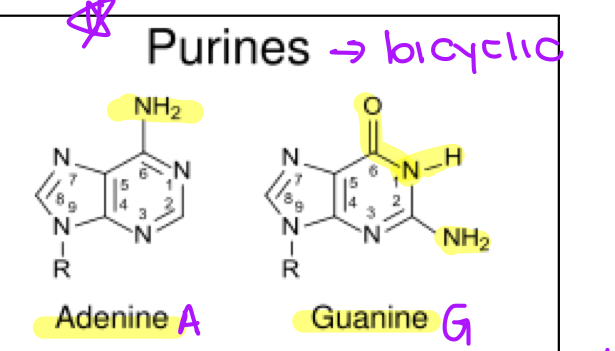
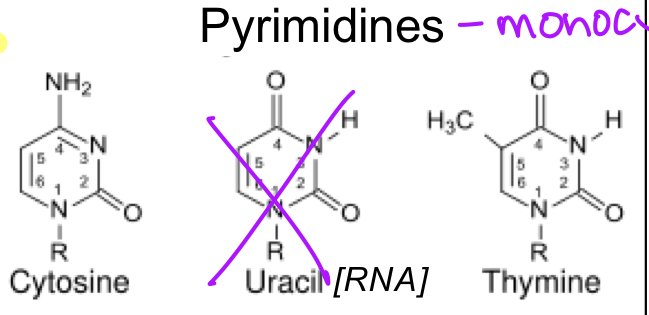
purines
bicyclic bases
adenine
guanine
pyrimidines
monocyclic bases
cytosine
thymine
uracil
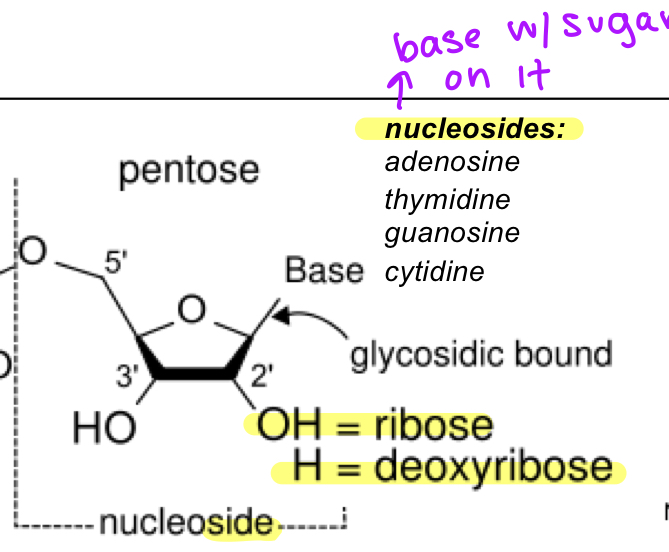
nucleosides
base with a pentose(sugar)
adenosine
thymidine
guanosine
cytidine
w OH is ribose
w H is deoxyribose
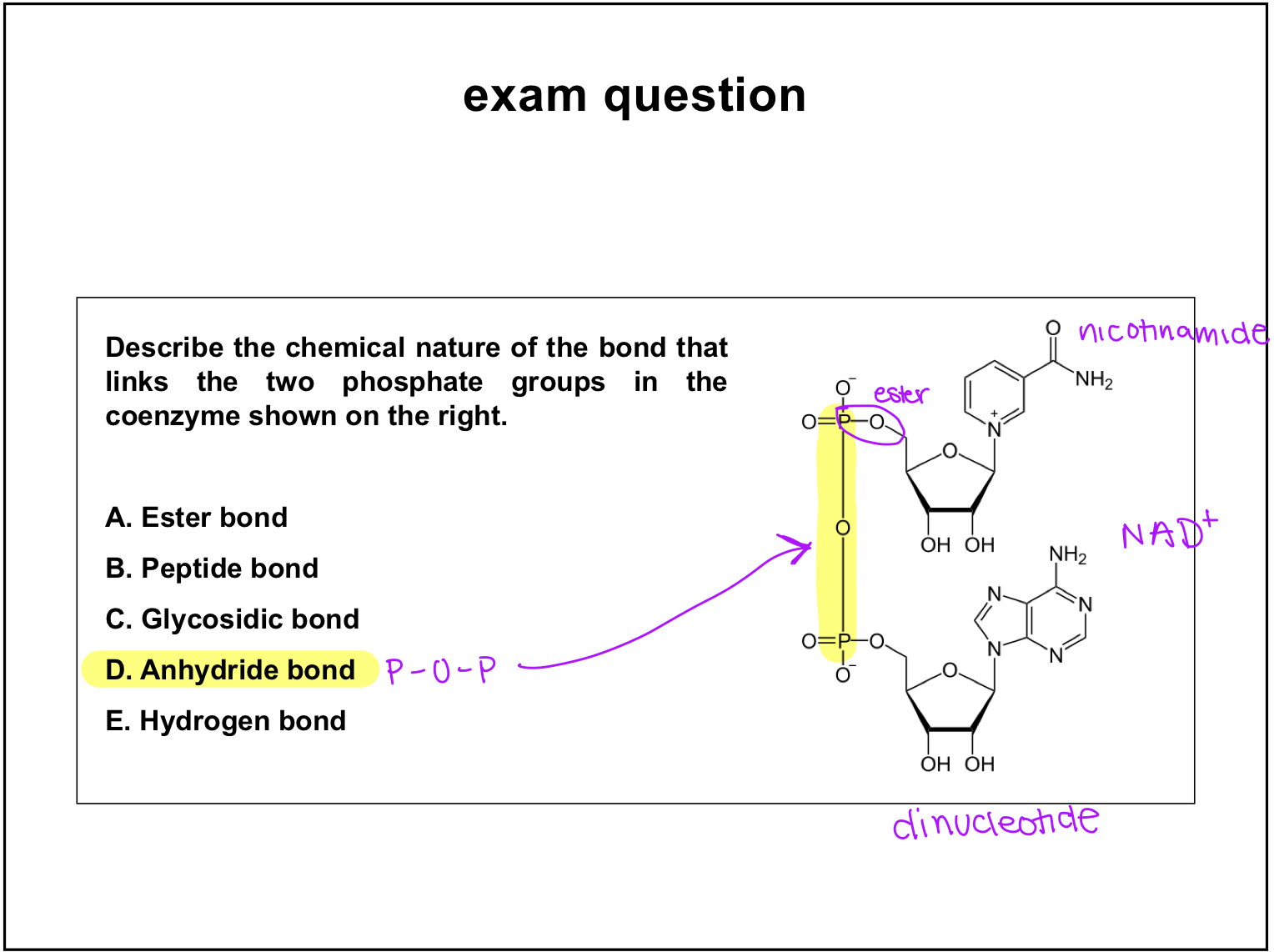
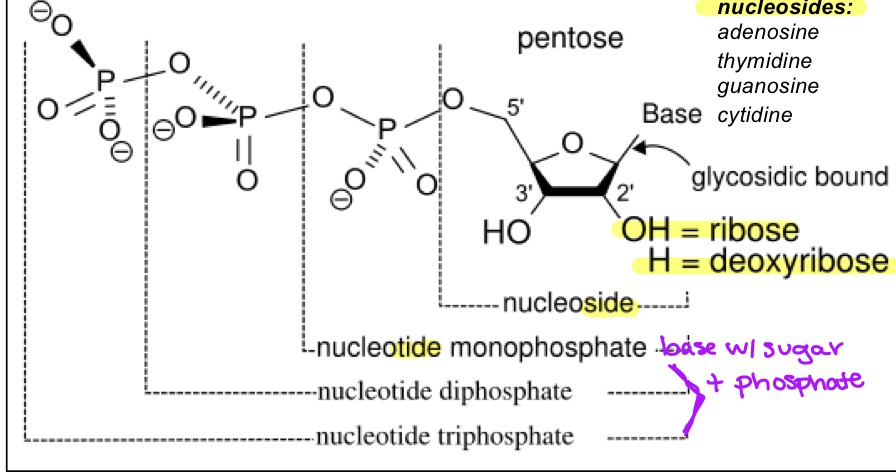
nucleotide
base+sugar+phosphate group/s
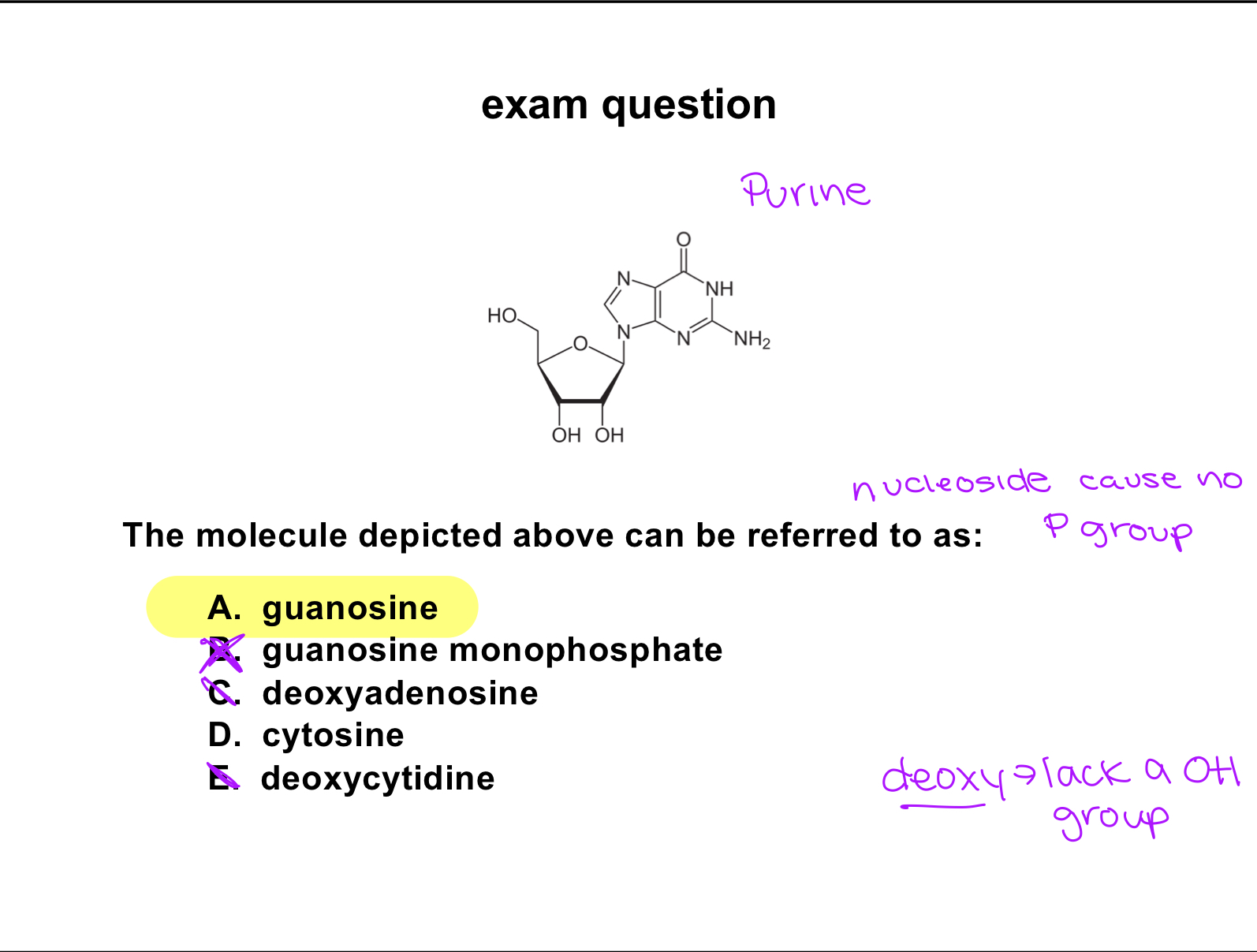
exam question
exam question
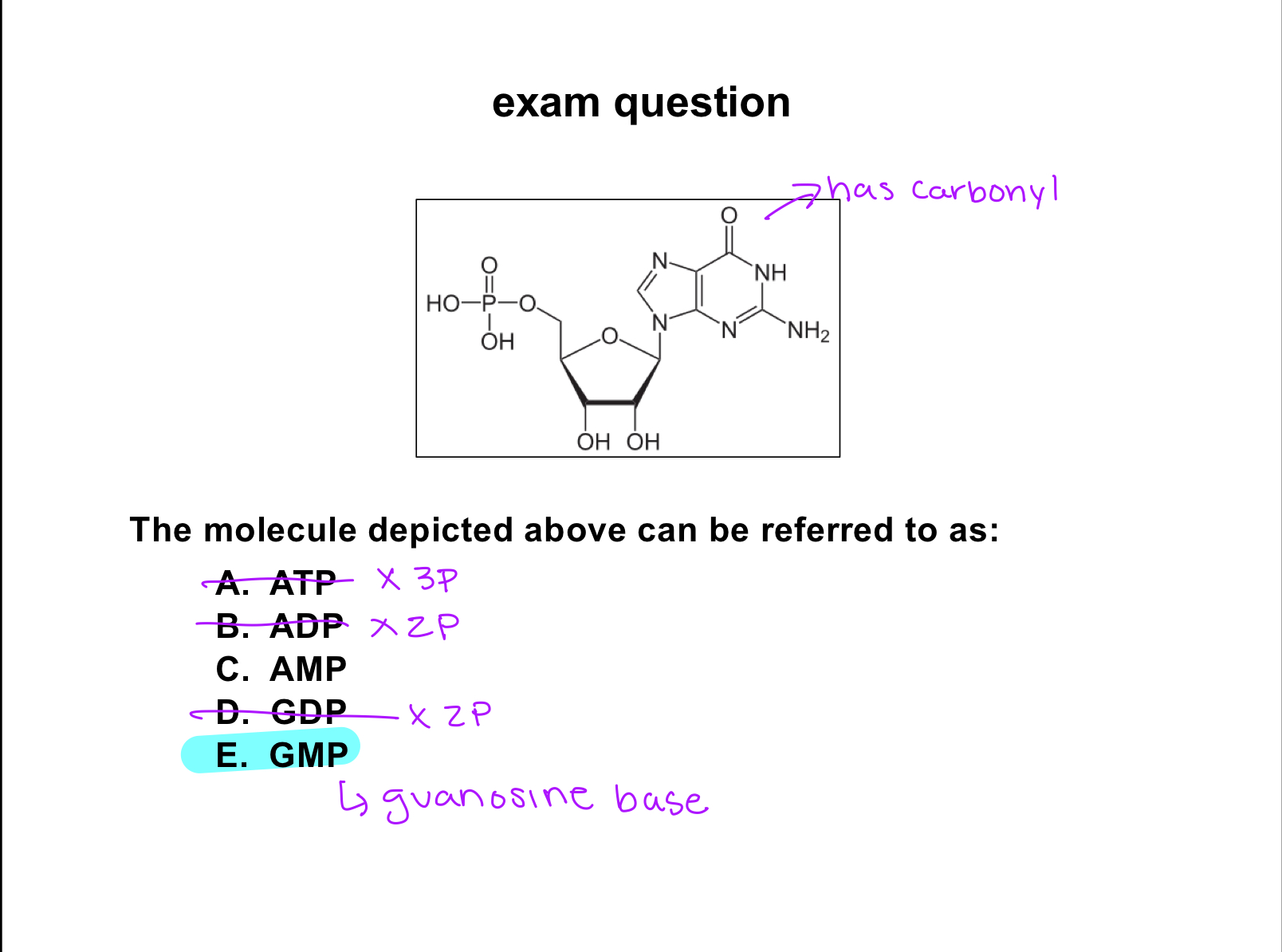
exam question
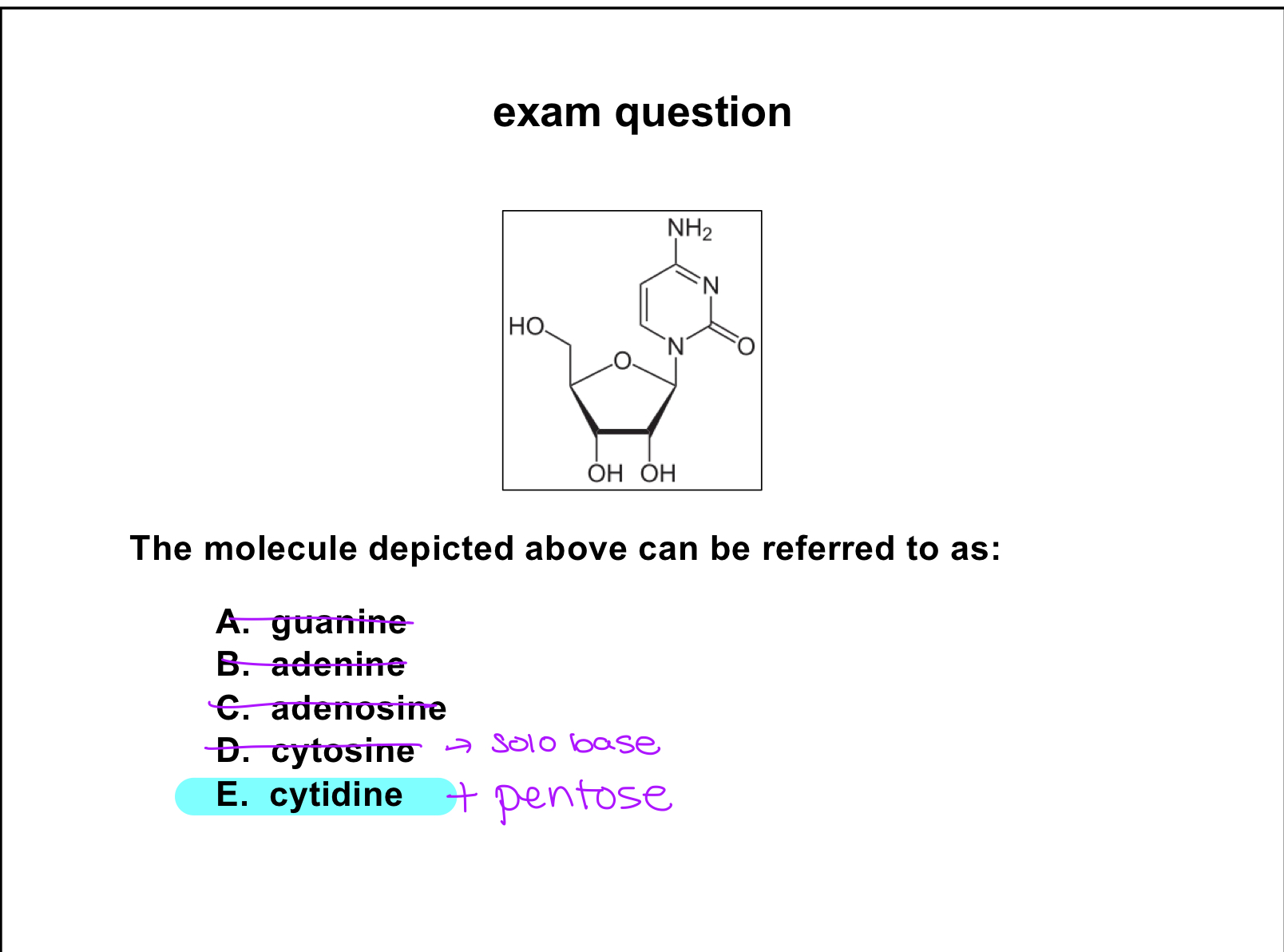
exam question