3.1 Biological molecules
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
-When monomers join together, they form polymers, which are long chains made up of multiple monomer units.
-Monosaccharides, amino acids and nucleotides are examples of monomers.
● 2 molecules join together
● Forming a chemical bond
● Releasing a water molecule
● 2 molecules separated
● Breaking a chemical bond
● Using a water molecule
-Glucose, galactose and fructose are common monosaccharides.
Draw structure of alpha glucose
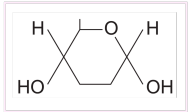
Draw structure of Beta glucose
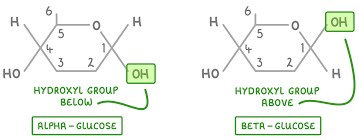
Describe the difference between the structure of α-glucose and β-glucose
● Isomers - same molecular formula but differently arranged atoms
● OH group is below carbon 1 in α-glucose but above carbon 1 in β-glucose
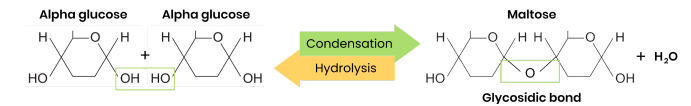
What are disaccharides and how are they formed?
● Two monosaccharides joined together with a glycosidic bond
● Formed by a condensation reaction, releasing a water molecule
Formed by the condensation of two glucose molecules.
Created by the condensation of glucose and fructose.
Formed by the condensation of glucose and galactose.
Draw a diagram to show how two monosaccharides are joined together
What are polysaccharides and how are they formed?
● Many monosaccharides joined together with glycosidic bonds
● Formed by many condensation reactions, releasing many water molecules
-Cellulose formed by the condensation of beta glucose.
How is it formed (Glycogen)
● Polysaccharide made of α-glucose
● 1,4- and 1,6-glycosidic bonds → branched
● Branched → compact / fit more molecules in small area
● Branched → more ends for faster hydrolysis → release glucose for respiration to make ATP for energy release
● Large, insoluble polysaccharide molecule → can’t leave cell / cross cell membrane
● Insoluble in water → water potential of cell not affected (no osmotic effect)
● Helical → compact for storage in cell
● Large, insoluble polysaccharide molecule → can’t leave cell / cross cell membrane
● Insoluble in water → water potential of cell not affected (no osmotic effect)
● Provides strength and structural support to plant / algal cell walls
● Polysaccharide of β-glucose
● 1,4-glycosidic bonds so forms straight, unbranched chains
● Chains linked in parallel by hydrogen bonds, forming microfibrils
Explain how the structure of cellulose relates to its function
● Every other β-glucose molecule is inverted in a long, straight, unbranched chain
● Many hydrogen bonds link parallel strands (crosslinks) to form microfibrils (strong fibres)
● Hydrogen bonds are strong in high numbers
● So provides strength to plant cell walls
-Add food sample to test tube
-Add benedicts reagent
-Heat sample gently
-If reducing sugar is present, solution will turn from blue to brick red.
-Add a few drops of iodine solution (iodine dissolved in potassium iodide) to the sample.
-Observe the colour change:
-If starch is present, the iodine solution will change from orange-brown to blue-black.
1. Do Benedict’s test (as above) and stays blue / negative
2. Heat in a boiling water bath with acid (to hydrolyse into reducing sugars)
3. Neutralise with alkali (eg. sodium bicarbonate)
4. Heat in a boiling water bath with Benedict’s solution
5. Positive result = green / yellow / orange / red precipitate
Name two groups of lipid
Triglycerides and phospholipids
Describe the difference between saturated and unsaturated fatty acids
● Saturated - no C=C double bonds in hydrocarbon chain → all carbons fully saturated with hydrogen
● Unsaturated - one or more C=C double bond in hydrocarbon chain (creating a bend / kink)
How does triglyceride properties relate to its function
● High ratio of C-H bonds to carbon atoms in hydrocarbon chain
○ So used in respiration to release more energy than the same mass of carbohydrates
● Hydrophobic / non-polar fatty acids so insoluble in water (clump together as droplets, tails inwards)
○ So no effect on water potential of cell (or can be used for waterproofing)
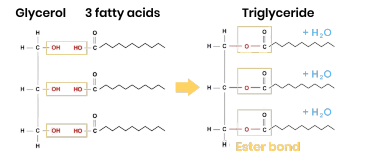
Triglycerides formation
● 1 glycerol molecule and 3 fatty acids
● 3 condensation reactions
● Removing 3 water molecules
● Forming 3 ester bonds
Structure: Phospholipids consist of a glycerol molecule attached to two fatty acids and a phosphate group. The phosphate group makes the head of the phospholipid hydrophilic (attracted to water), while the fatty acid tails are hydrophobic (repelled by water).
Form a bilayer in cell membrane, allowing diffusion of lipid-soluble (non-polar) or very small substances and restricting movement of water-soluble (polar) or larger substances
How does phospholipid Structure Related to Their Properties
● Phosphate heads are hydrophilic
○ Attracted to water so point to water (aqueous environment) either side of membrane
● Fatty acid tails are hydrophobic
○ Repelled by water so point away from water / to interior of membrane
-A cloud white emulsion occurs if lipid present
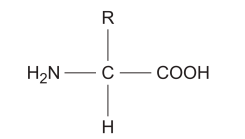
An amino group (NH₂) A carboxyl group (-COOH) A variable R group, which is a side chain that differs in each amino acid and determines its specific properties.
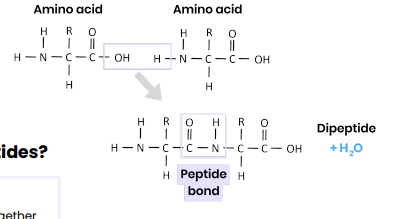
● Condensation reaction
● Removing a water molecule
● Between carboxyl / COOH group of one and amine / NH2 group of another
● Forming a peptide bond
Sequence of amino acids in a polypeptide chain, joined by peptide bonds
-This folding creates two common shapes:
•Alpha-helix: A coiled structure.
•Beta-pleated sheet: A folded, sheet-like structure.
-The slight positive charge of hydrogen in the NH group and the slight negative charge of oxygen in the C=O group create weak hydrogen bonds, stabilising these structures.
•Disulfide Bridges: Strong covalent bonds between sulfur atoms in the amino acid cysteine, resistant to breaking.
•Ionic Bonds: Form between charged amino acid side chains. These are weaker than disulfide bridges and can be disrupted by changes in pH.
•Hydrogen Bonds: Abundant but weaker bonds that contribute to stability.
● More than one polypeptide chain
● Formed by interactions between polypeptides (hydrogen bonds, ionic bonds, disulfide bridges)
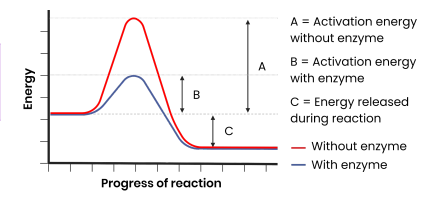
How do enzymes act as biological catalysts?
● Each enzyme lowers activation energy of reaction it catalyses
● To speed up rate of reaction
-The substrate is the molecule that binds to the enzyme's active site.
-Enzyme specificity means that each enzyme is specific to one substrate, as only that substrate has the complementary shape to fit into the active site.
-The substrate fits precisely into the active site, forming an enzyme-substrate complex, which allows the reaction to proceed.
-This model explains enzyme specificity, where each enzyme is specific to one type of reaction.
1. Substrate binds to (not completely complementary) active site of enzyme
2. Causing active site to change shape (slightly) so it is complementary to its substrate
3. So enzyme-substrate complex forms
4. Causing bonds in substrate to bend / distort, lowering activation energy
Explain the specificity of enzymes
● Specific tertiary structure determines shape of active site
○ Dependent on sequence of amino acids (primary structure)
● Active site is complementary to a specific substrate
● Only this substrate can bind to active site, inducing fit and forming an enzyme-substrate complex
Describe and explain the effect of temperature on the rate of enzyme-controlled reactions
● As temperature increases to optimum, rate of reaction increases
○ More kinetic energy
○ So more enzyme-substrate complexes form
● As temperature exceeds optimum, rate of reaction decreases
○ Enzymes denature - tertiary structure and active site change shape
○ As hydrogen / ionic bonds break
○ So active site no longer complementary
○ So fewer enzyme-substrate complexes form
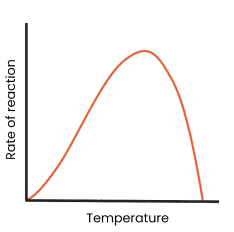
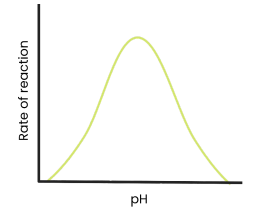
Describe and explain the effect of pH on the rate of enzyme-controlled reactions
● As pH increases / decreases above / below an optimum, rate of reaction decreases
○ Enzymes denature - tertiary structure and active site change shape
○ As hydrogen / ionic bonds break
○ So active site no longer complementary
○ So fewer enzyme-substrate complexes form
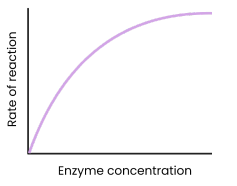
Describe and explain the effect of enzyme concentration on the rate of enzyme-controlled reactions
● As enzyme concentration increases, rate of reaction increases
○ Enzyme concentration = limiting factor (excess substrate)
○ More enzymes so more available active sites
○ So more enzyme-substrate complexes form
● At a certain point, rate of reaction stops increasing / levels off
○ Substrate concentration = limiting factor (all substrates in use)
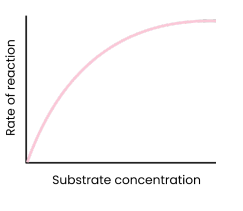
Describe and explain the effect of substrate concentration on the rate of enzyme-controlled reactions
● As substrate concentration increases, rate of reaction increases
○ Substrate concentration = limiting factor (too few substrate molecules to occupy all active sites)
○ More enzyme-substrate complexes form
● At a certain point, rate of reaction stops increasing / levels off
○ Enzyme concentration = limiting factor
○ As all active sites saturated / occupied (at a given time)
Describe and explain the effect of concentration of competitive inhibitors on the rate of enzyme-controlled reactions
● As concentration of competitive inhibitor increases, rate of reaction decreases
○ Similar shape to substrate
○ Competes for / binds to / blocks active site
○ So substrates can’t bind
○ So fewer enzyme-substrate complexes form
● Increasing substrate concentration reduces effect of inhibitors (dependent on relative concentrations of substrate and inhibitor)
Describe and explain the effect of concentration of non-competitive inhibitors on the rate of enzyme-controlled reactions
● As concentration of non-competitive inhibitor increases, rate of reaction decreases
○ Binds to site other than the active site (allosteric site)
○ Changes enzyme tertiary structure / active site shape
○ So active site no longer complementary to substrate
○ So substrates can’t bind
○ So fewer enzyme-substrate complexes form
● Increasing substrate concentration has no effect on rate of reaction as change to active site is permanent
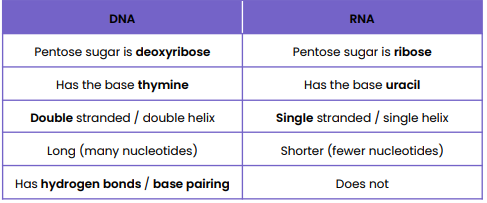
Holds genetic information which codes for polypeptides (proteins)
DNA to the ribosome.
•tRNA (Transfer RNA): Brings amino acids to the ribosome to build proteins.
•rRNA (Ribosomal RNA): Combines with proteins to form ribosomes, the site of protein synthesis.
Transfers genetic information from DNA to ribosomes
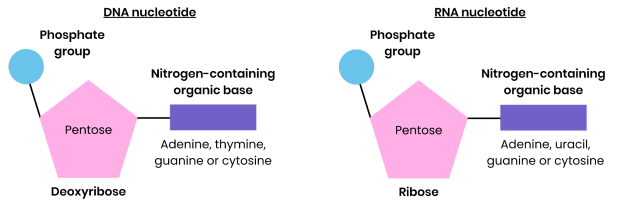
Draw and label a DNA nucleotide and an RNA nucleotide
Name the two types of molecule from which a ribosome is made
RNA and proteins
2.) A phosphate group, and
3.) A nitrogen-containing organic base.
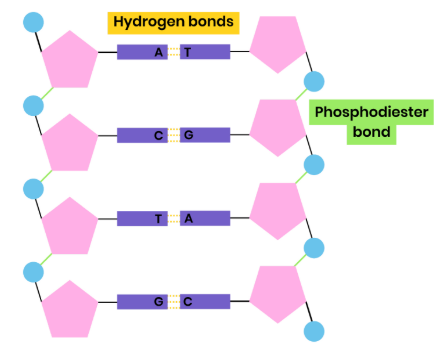
Structure of DNA Nucleotide
● Polymer of nucleotides (polynucleotide)
● Each nucleotide formed from deoxyribose, a phosphate group and a nitrogen-containing organic base
● Phosphodiester bonds join adjacent nucleotides
● 2 polynucleotide chains held together by hydrogen bonds
● Between specific complementary base pairs - adenine / thymine and cytosine / guanine
● Double helix
Structure of RNA Nucleotide
● Polymer of nucleotides (polynucleotide)
● Each nucleotide formed from ribose, a phosphate group and a nitrogen-containing organic base
● Bases - uracil, adenine, cytosine, guanine
● Phosphodiester bonds join adjacent nucleotides
● Single helix
● Condensation reactions, removing water molecules
● Between phosphate group of one nucleotide and deoxyribose / ribose of another
● Forming phosphodiester bonds
Why did many scientists initially doubt that DNA carried the genetic code?
The relative simplicity of DNA - chemically simple molecule with few components
Suggest how the structure of DNA relates to its functions
● Two strands → both can act as templates for semi-conservative replication
● Hydrogen bonds between bases are weak → strands can be separated for replication
● Complementary base pairing → accurate replication
● Many hydrogen bonds between bases → stable / strong molecule
● Double helix with sugar phosphate backbone → protects bases / hydrogen bonds
● Long molecule → store lots of genetic information (that codes for polypeptides)
● Double helix (coiled) → compact
Suggest how you can use incomplete information about the frequency of bases on DNA strands to find the frequency of other bases
1. % of adenine in strand 1 = % of thymine in strand 2 (and vice versa)
2. % of guanine in strand 1 = % of cytosine in strand 2 (and vice versa) Because of specific complementary base pairing between 2 strands
Why is semi-conservative replication important?
Ensures genetic continuity between generations of cells
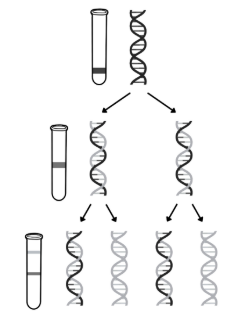
Describe the work of Meselson and Stahl in validating the Watson-Crick model of semi-conservative DNA replication
1. Bacteria grown in medium containing heavy nitrogen (15N) so nitrogen is incorporated into DNA bases
○ DNA extracted & centrifuged → settles near bottom, as all DNA molecules contain 2 ‘heavy’ strands
2. Bacteria transferred to medium containing light nitrogen (14N) and allowed to divide once
○ DNA extracted & centrifuged → settles in middle, as all DNA molecules contain 1 original ‘heavy’ and 1 new ‘light’ strand
3. Bacteria in light nitrogen (14N) allowed to divide again
○ DNA extracted & centrifuged → half settles in middle, as contains 1 original ‘heavy’ and 1 new ‘light’ strand; half settles near top, as contains 2 ‘light’ strands
Describe the process of semi-conservative DNA replication
1. DNA helicase breaks hydrogen bonds between complementary bases, unwinding the double helix
2. Both strands act as templates
3. Free DNA nucleotides attracted to exposed bases and join by specific complementary base pairing
4. Hydrogen bonds form between adenine-thymine and guanine-cytosine
5. DNA polymerase joins adjacent nucleotides on new strand by condensation reactions
6. Forming phosphodiester bonds
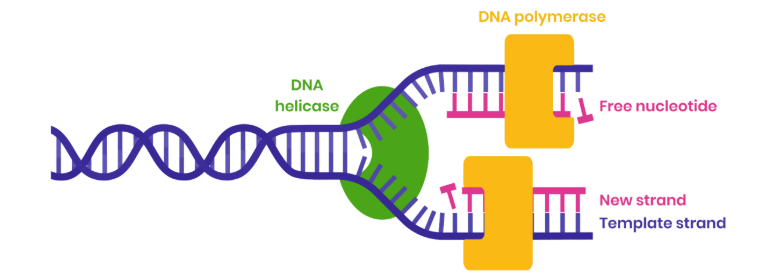
Use your knowledge of enzyme action to suggest why DNA polymerase moves in opposite directions along DNA strands
● DNA has antiparallel strands
● So shapes / arrangements of nucleotides on two ends are different
● DNA polymerase is an enzyme with a specific shaped active site
● So can only bind to substrate with complementary shape (phosphate end of developing strand)
Name the two scientists who proposed models of the chemical structure of
DNA and of DNA replication
Watson and Crick
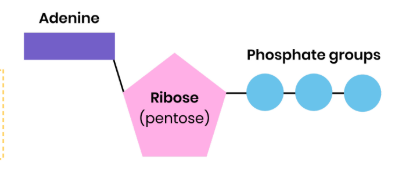
Describe the structure of ATP
● Ribose bound to a molecule of adenine (base) and 3 phosphate groups
● Nucleotide derivative (modified nucleotide)
Describe how ATP is broken down
● ATP (+ water) → ADP (adenosine diphosphate) + Pi (inorganic phosphate)
● Hydrolysis reaction, using a water molecule
● Catalysed by ATP hydrolase (enzyme)
Describe how ATP is resynthesised in cells
● ADP + Pi → ATP (+ water)
● Condensation reaction, removing a water molecule
● Catalysed by ATP synthase (enzyme)
● During respiration and photosynthesis
Describe how ATP is resynthesised in cells
● ADP + Pi → ATP (+ water)
● Condensation reaction, removing a water molecule
● Catalysed by ATP synthase (enzyme)
● During respiration and photosynthesis
Explain how hydrogen bonds occur between water molecules
● Water is polar molecule
● Slightly negatively charged oxygen atoms attract slightly positively charged hydrogen atoms of other water molecules
-The oxygen atom is more electronegative and attracts the electrons more strongly than the hydrogen atoms.
-As a result, the oxygen end of the molecule is slightly negative, and the hydrogen end is slightly positive.
-This polarity allows water molecules to form hydrogen bonds with each other, causing them to stick together.
Properties of water: Metabolite
Used in condensation / hydrolysis / photosynthesis / respiration
Properties of water: Solvent
1. Allows metabolic reactions to occur (faster in solution)
2. Allows transport of substances eg. nitrates in xylem, urea in blood
Properties of water: High Specific Heat Capacity
● Buffers changes in temperature
● As can gain / lose a lot of heat / energy without changing temperature
1. Good habitat for aquatic organisms as temperature more stable than land
2. Helps organisms maintain a constant internal body temperature
•This is important for processes like the transport of water in plants (e.g., through the xylem).
•Cohesion supports the column of water, allowing it to move upwards through narrow tubes.
-High surface tension at the water-air boundary is due to the strong cohesion between water molecules, allowing small organisms (e.g., water striders) to walk on water without sinking.
Large latent heat of vaporisation
● Allows effective cooling via evaporation of a small volume (eg. sweat)
● So helps organisms maintain a constant internal body temperature
Where are inorganic ions found in the body?
In solution in cytoplasm and body fluid, some in high concentrations and others in very low concentrations
Describe the role of hydrogen ions
● Maintain pH levels in the body → high concentration = acidic / low pH
● Affects enzyme rate of reaction as can cause enzymes to denature
Describe the role of iron ions
● Component of haem group of haemoglobin
● Allowing oxygen to bind / associate for transport as oxyhaemoglobin
Sodium ions
1. Involved in co-transport of glucose / amino acids into cells
2. Involved in action potentials in neurons
3. Affects water potential of cells / osmosis
Phosphate ions
1. Component of nucleotides, allowing phosphodiester bonds to form in DNA / RNA
2. Component of ATP, allowing energy release
3. Phosphorylates other compounds making them more reactive
4. Hydrophilic part of phospholipids, allowing a bilayer to form