science | at1 - the periodic table groups
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
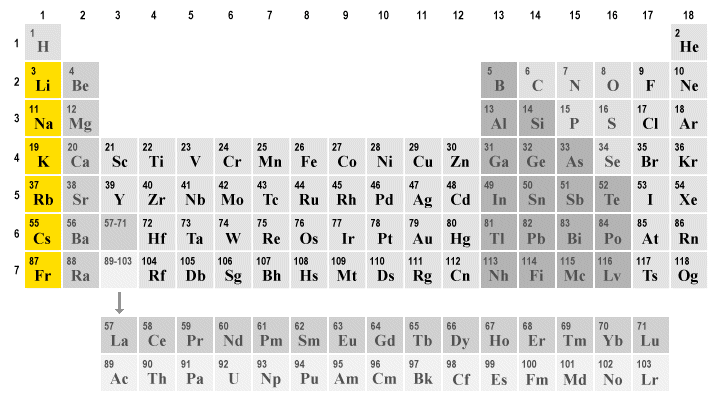
alkali metals (group 1)
they have typical metal properties. they are the most reactive metals but they are too reactive to be found in their pure form. they display extreme chemical behaviour as they react violently with water by exploding. because of this, they need to be handled carefully by being stored in a bottle of kerosene to not come into contact with air or water. they are soft enough to be cut with a knife
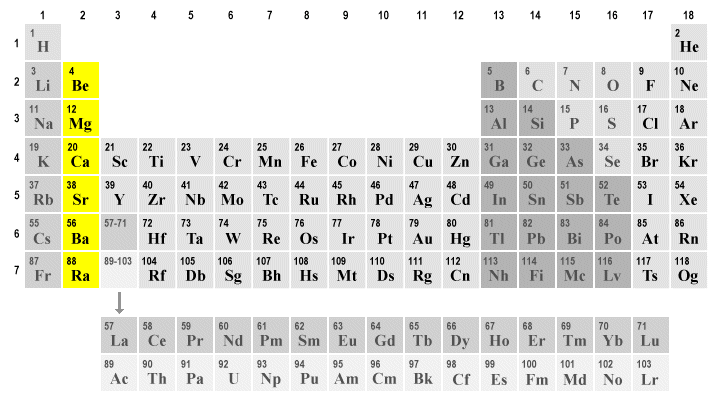
alkaline earth metals (group 2)
they have high melting points and they act similar to group 1 but are slightly less reactive. they are slightly harder than alkali metals and an alkaline solution can be formed when a metal oxide is dissolved in water.
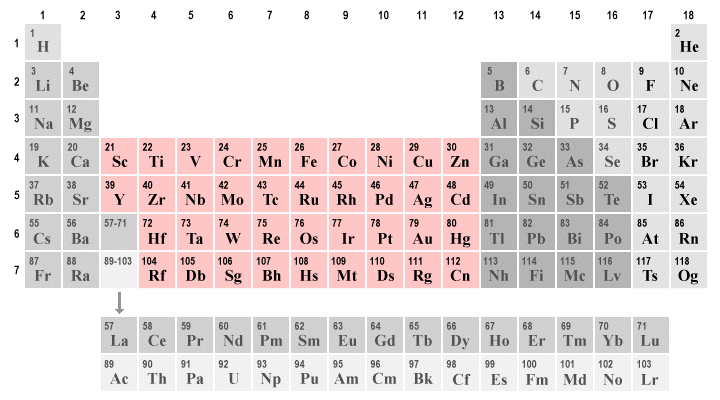
transition metals (groups 3 to 12)
includes the lanthanides and actinides. generally, transition metals are lustrous, silvery, hard and good conductors of electricity and heat. some elements are more malleable than others and properties between individual elements may vary greatly
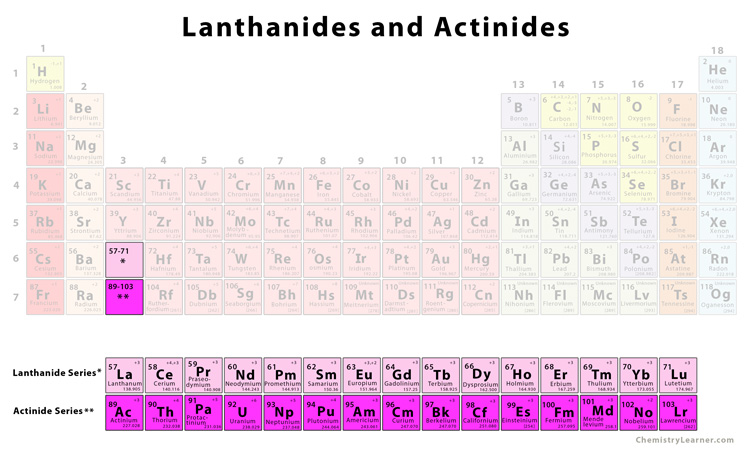
lanthanides and actinides
they are referred to as rare earth metals. they are all radioactive and they have properties which set them apart from all the elements despite them being transition metals
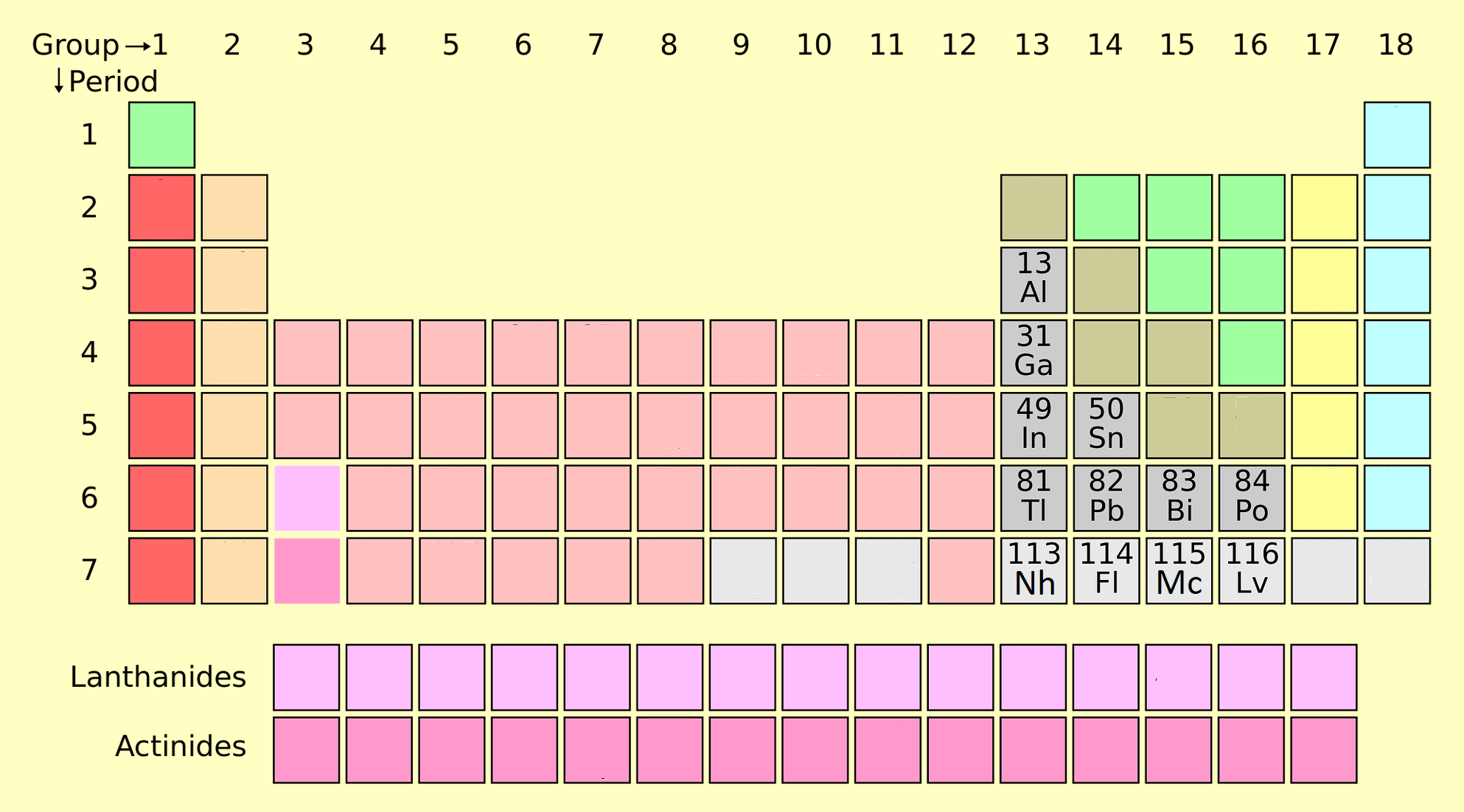
post transition metals
has some properties of transition metals but are softer and conduct more poorly than them
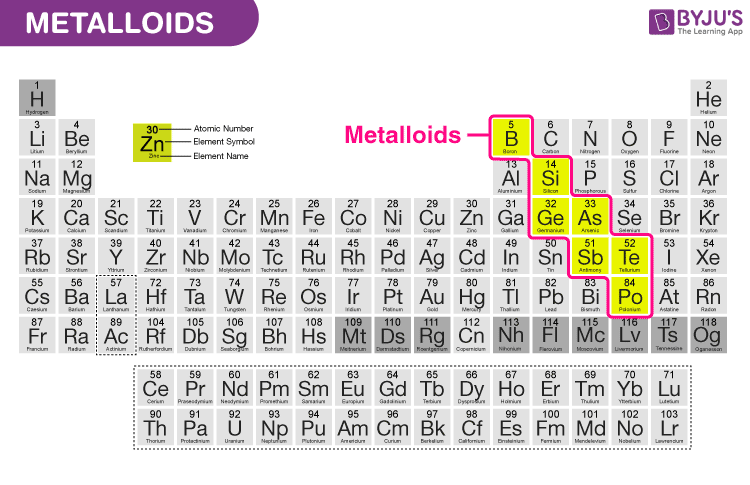
metalloids
they behave as semiconductors rather than conducters
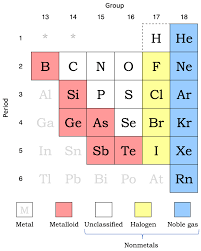
non metals
have low melting and boiling points and they are found in the form of liquids and gases. they are very brittle and have low ductility and do not conduct heat or electricity well. they have no metallic lustre and do not reflect light.
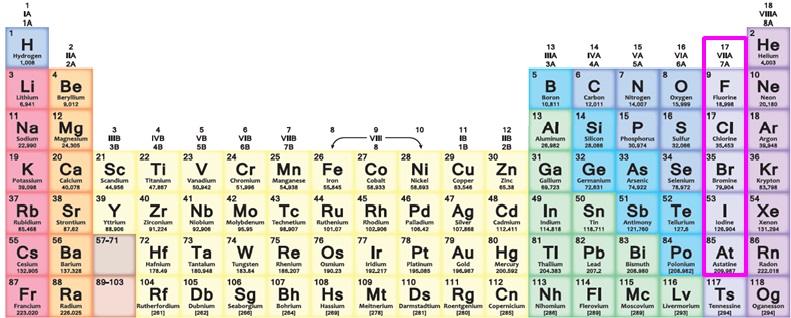
halogens (group 17)
present in the environment as compounds rather than pure elements meaning they all form molecules that are made up of 2 atoms. they are not found in nature in their pure form but they are found in various salts. they get bigger and less reactive as you move down a group.
noble gases (group 18)
they are very chemically stable and only react under extreme circumstances. they are colourless, odourless and occur naturally in the atmosphere. as you go down the group, the elements become more denser