STUDY GUIDE EXAM 1: Covering- Thiamin, Riboflavin, Niacin, Pantothenic Acid, Biotin, (pyridoxine not added yet)
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
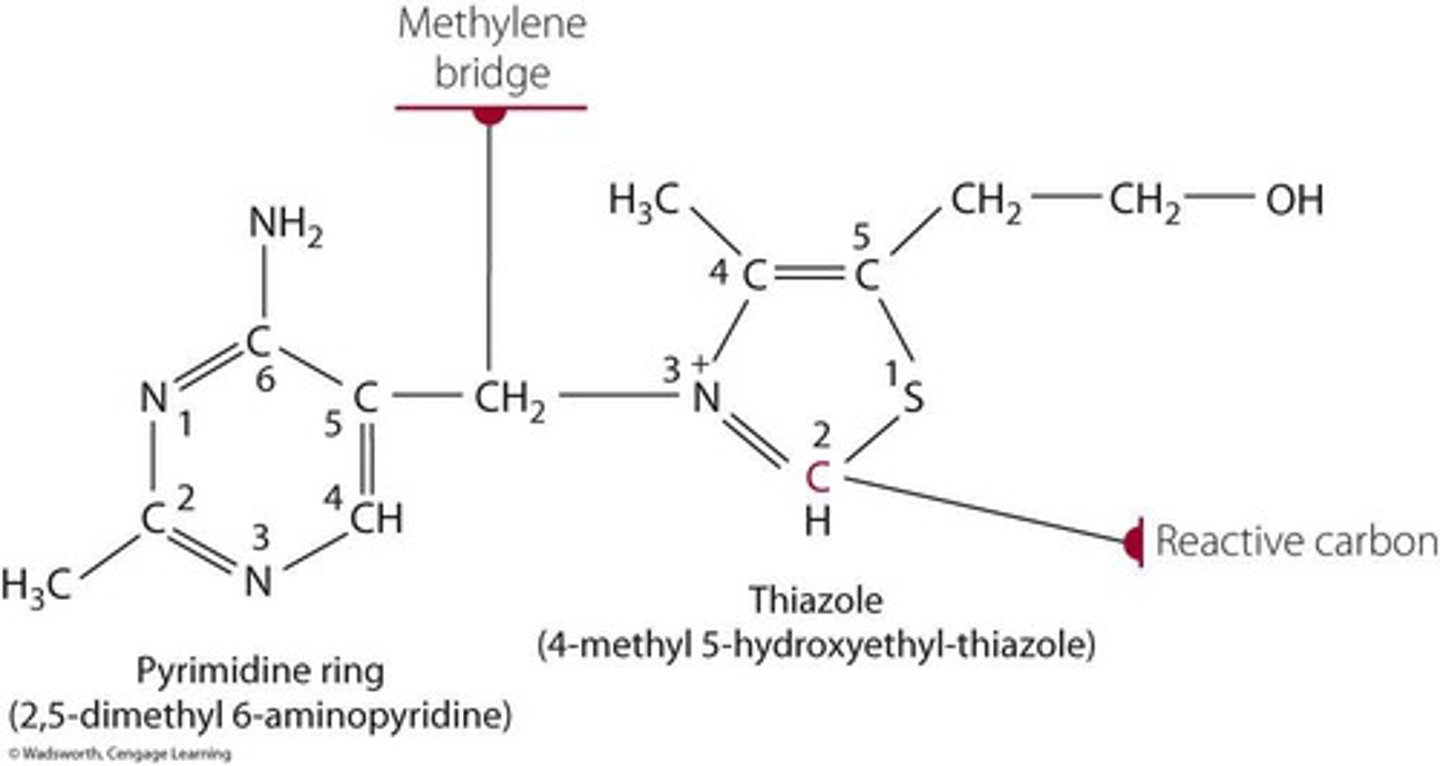
structure of thiamin
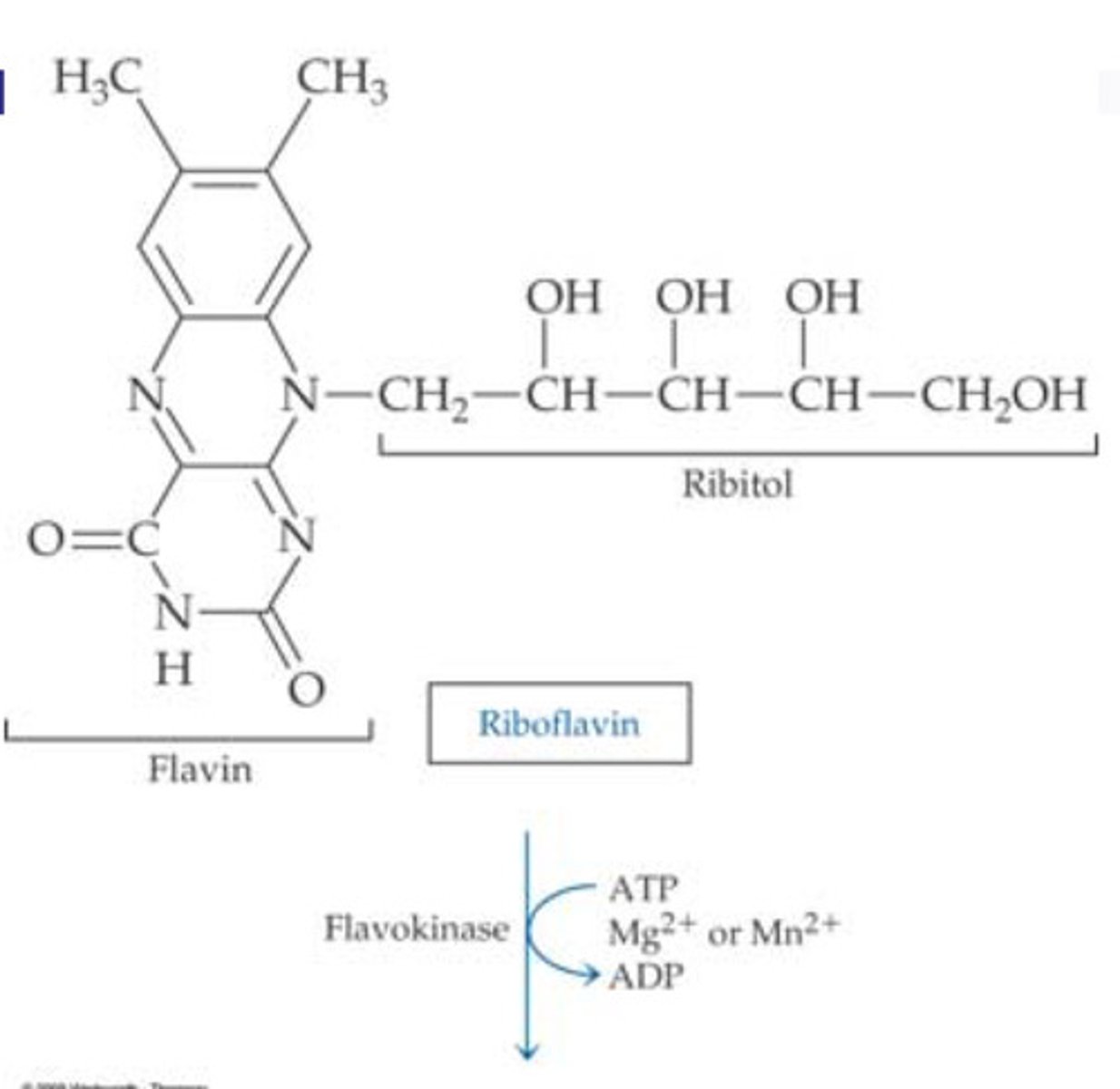
structure of riboflavin
structure of niacin
nicotinic acid and nicotinamide (COHN2)

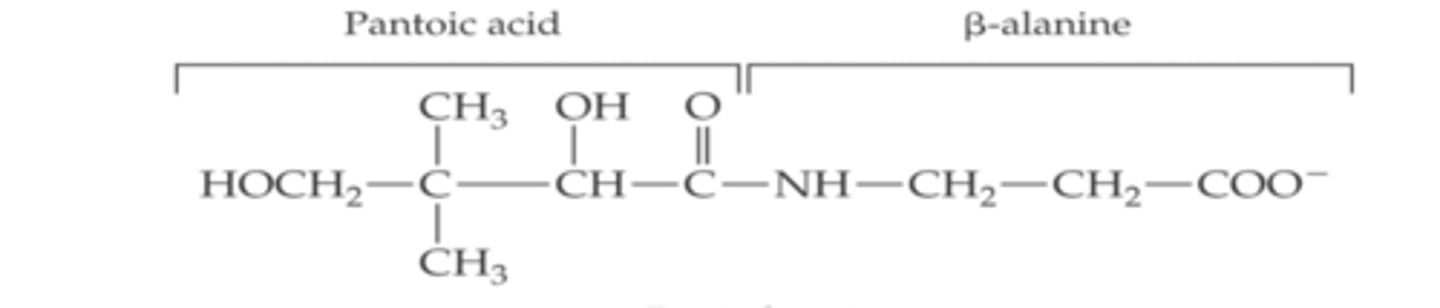
structure of Pantothenic acid
structure of thiamin description
two rings connected by a bridge
structure of riboflavin description
3 rings on top of each other with chain sticking out middle
structure of niacin
2 separate rings
structure of pantothenic acid
1 long chain
Active form of thiamin (functional form)
-Thiamin Diphosphate (TDP),
- primarily found in red blood cells and acts as coenzyme in energy transformation and nerve transmission processes.
active form of niacin (functional form)
- Nicotinamide Adenine Dinucleotide (NAD+) and
- Nicotinamide Adenine Dinucleotide Phosphate (NADP+)
- essential in oxidative and reductive biosynthesis reactions
active form of riboflavin (functional form)
-Flavin Mononucleotide (FMN) (requires flavokinase)
- Flavin Adenine Dinucleotide (FAD) (requires FAD synthetase)
- involved in redox reactions and energy metabolism
-both require Either magnesium (Mg2+) or manganese (Mn2+) ions
active form of Pantothenic acid (functional form)
- Coenzyme A (CoA)
- 4'-Phosphopantetheine
-essential in energy metabolism, fatty acid synthesis, and acetylation processes
NE calculation
- 1 mg Niacin Equivalent (NE) = 60 mg of tryptophan = 1 mg niacin.
-For example, 60 g of high-quality protein provides 10 mg NE.
Factors affecting vitamin availability from food for Niacin
-poor protein diets
-medication use
- prolonged treatment with isoniazid (anti-tuberculosis drug),
-malabsorptive disorders.
Factors affecting vitamin availability from food for pantothenic acid
- When biotin concentration low Na dependent active multivitamin transporter (SMVT) is used in heart muscle, brain & liver
- when biotin concentration high passive diffusion used (occurs in other tissues)
Factors affecting vitamin availability from food for riboflavin
- inhibited by alcohol and divalent metals (Cu, Zn, Fe, Mn)
- enhanced by bile.
- Animal sources are better absorbed than plant sources.
Factors affecting vitamin availability from food for thiamin
- Antithiamin factors like thiaminases (raw fish) and polyhydroxyphenols (coffee, tea) reduce bioavailability.
- Phosphorylated thiamin must be hydrolyzed to the free form.
Functions of thiamin
energy transformation, the pentose phosphate pathway, and nerve transmission.
Functions of niacin
-supports oxidative reactions, reductive biosynthesis, ADP-ribosylation for DNA repair, and cholesterol and fatty acid synthesis.
functions of pantothenic acid
Critical for coenzyme A synthesis (TCA CYCLE) fatty acid metabolism, acetylation processes, and synthesis of cholesterol, heme, and other biomolecules.
functions of riboflavin
Acts as a cofactor for flavoproteins, supports redox reactions, and is vital for energy production and antioxidant functions.
metabolism of niacin
- metabolized in liver
-converted into NAD+ and NADP+
- excreted as methylated products in urine.
metabolism of pantothenic acid
- CoA hydrolyzed to pantothenic acid
- absorbed via active or passive transport
- Hydrolyzed in digestion to pantothenic acid, absorbed in the jejunum.
-Uptake via SMVT (sodium-dependent multivitamin transporter).
-excreted in urine.
metabolism of riboflavin
- Absorbed as FMN or FAD in cells
-metabolized to coenzyme forms
-excreted in urine.
metabolism of thiamin
-Free thiamin absorbed in jejunum via active transport or diffusion
- converted to TDP in cells
- Excess is excreted in urine.
Nutritional assessment of thiamin
- Assess through urinary excretion or red blood cell transketolase activity
- deficiency indicated by >25% increased activity after thiamin addition
Nutritional assessment of riboflavin
- Measure erythrocyte glutathione reductase activity
- (AC > 1.4 = deficiency)
- urinary riboflavin levels <40 µg/day = deficiency
Nutritional assessment of pantothenic acid
- Urinary excretion <1 mg/day indicates deficiency
- Plasma or serum levels can also be measured.
nutritional assessment of niacin
- Assess via urinary metabolites or dietary intake of niacin equivalents (NE).
Deficiency & Toxicity of thiamin
- Deficiency causes beriberi (dry, wet, or acute) and Wernicke's encephalopathy.
- Toxicity is rare as excess thiamin is excreted.
Deficiency and Toxicity of niacin
- Deficiency leads to pellagra (3 Ds: dermatitis, diarrhea, dementia).
- Toxicity from high doses causes flushing, liver damage, and glucose intolerance.
Deficiency & Toxicity of pantothenic acid
- Deficiency causes burning feet syndrome, fatigue, and weakness.
-Toxicity is rare.
Deficiency & Toxicity of riboflavin
- Deficiency (ariboflavinosis) results in cheilosis, glossitis, and neuropathy.
-Toxicity is not reported with high doses.
Factors affecting vitamin availability from food for biotin
- Avidin (found in raw egg whites) prevents biotin absorption.
- Cooking eggs denatures avidin, allowing biotin absorption.
-Biotin can be synthesized by colonic bacteria, but contribution to overall needs is uncertain.
Functions of biotin
- Essential coenzyme for carboxylase enzymes in metabolism.
-Required for fatty acid synthesis and gluconeogenesis.
-Helps regulate gene expression.
-Plays a role in cell signaling and chromatin structure.
Nutritional assessment of biotin
- Urinary excretion of less than 6 µg/day may indicate deficiency.
- Blood plasma levels less than 200 pg/mL suggest low biotin status.
-AI intake =19 and up 30 µg/day
Deficiency & Toxicity of biotin
symptoms: Lethargy, depression, hallucination Muscle pain, anorexia, nausea, Hair loss (alopecia), red scaly dermatitis
Toxicity: No reported toxic effects from high doses.
Active form of biotin (functional form)
- Biotin (B7) as a coenzyme binds to carboxylases via lysine
-When attached, forms HOLOCARBOXYLASE, the active enzyme (ATP required for this step)
- Acetyl-CoA is key allosteric activator
-Binds to specific site on PC (pyruvate carboxylase)
-Causes conformational change that increases enzyme activity
Metabolism of biotin
-Absorbed in jejunum via sodium-dependent multivitamin transporter (SMVT).
-Stored in small amounts in muscle, liver, and brain.
-Recycled by removal from carboxylases when needed.
-Excreted in urine mainly as bisnorbiotin, tetranorbiotin, biotin sulfoxide, and biotin sulfone.
biotin enzyme formation
1. ATP + bicarbonate ion → carbonic phosphoric anhydride ion
2. Reacts with biotin NH → transfers CO₂ to biotin
3. Biotin then attaches to lysine in the enzyme, forming a flexible long arm for catalytic function.
Enzymes requiring biotin as a cofactor
1. Pyruvate carboxylase → Converts pyruvate to oxaloacetate (TCA cycle & gluconeogenesis).
2. Acetyl-CoA carboxylase 1 & 2 → Converts acetyl-CoA to malonyl-CoA (fatty acid synthesis regulation).
3. Propionyl-CoA carboxylase → Converts propionyl-CoA to methylmalonyl-CoA (amino acid & odd-chain fatty acid metabolism).
4. Methylcrotonyl-CoA carboxylase → Breaks down leucine & isoprenoid compounds.
Biotin recycling
- Biotin can be recycled after its removal from carboxylase enzymes.
- Reduces the body's dietary biotin requirement.
Biotin deficiency impact on metabolism
Reduces pyruvate carboxylase activity → lower oxaloacetate levels → impairs TCA cycle & gluconeogenesis → leads to low ATP & glucose (fatigue & lethargy).
Biotin & Drug Interactions
1. Anticonvulsant drugs (primidone, carbamazepine)
- Reduce blood biotin levels.Increase urinary excretion of biotin metabolites.
2. Long-term sulfa drug use (antibiotics)
- May decrease bacterial synthesis of biotin in the gut.
Relationship between biotin & pantothenic acid
-both biotin & pantothenic acid use Sodium-Dependent Multivitamin Transporter (SMVT)
-High doses of biotin supplements can reduce pantothenic acid absorption from food becasue excessive biotin may block pantothenic acid uptake
Niacin synthesis from tryptophan
- Occurs in the liver via the kynurenine pathway.
- Requires riboflavin, iron, and vitamin B6 for conversion.
Niacin transportation & storage
-Transported from liver to all tissues
-Converted to coenzyme forms NAD+ and NADP+
- stored in liver as Coenzyme forms (NAD+ and NADP+)
-excess niacin intake excreted in urine
Coenzyme roles of Niacin
- NAD+: Used in catabolic reactions (electron & hydrogen ion acceptor).
- NADP+: Used in anabolic reactions (fatty acid and cholesterol synthesis).
NADH in energy production
- NAD+ is reduced to NADH during macronutrient breakdown.
- NADH donates electrons in the electron transport chain, generating ATP.
NADPH in biosynthesis
- Provides electrons for biosynthesis of macromolecules (proteins, carbs, fats).
- Found in high concentrations in liver and mammary glands due to fatty acid synthesis.
Niacin in antioxidant function
- Involved in the glutathione oxidation-reduction cycle, maintaining cellular redox balance.
Non-redox roles of niacin
- ADP-ribosylation reactions:
- DNA repair, replication, transcription
- G-protein activity
- Chromatin structure regulation
Niacin and cardiovascular health
- Nicotinic acid improves lipid profile:
- Increases HDL
- Decreases LDL
- Decreases apolipoprotein B
- Reduces heart disease risk
Niacin and drug interactions
1. Nicotinic acid + Lovastatin may lead to rhabdomyolysis.
2. Estrogen and estrogen containing oral contraceptives increase niacin synthesis, reducing dietary needs.
3. 5-Fluorouracil (chemotherapy drug) can cause pellagra, requiring niacin supplementation.
4. Isoniazid (anti-tuberculosis drug) increases niacin needs.
pantothenic acid Coenzyme A synthesis - Rate-limiting step
Phosphorylation of pantothenic acid by pantothenate kinase.
Pantothenic acid and disease treatment
-May improve wound healing.
- Pantethine (derivative) may help lower cholesterol.