Quiz 2/ Exam 2 BMS2
1/162
Earn XP
Description and Tags
Learning Objectives for Austin and Kearns
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
163 Terms
Pharmacokinetics
The study of how drugs are absorbed, distributed, metabolized, and eliminated by the body. It helps determine drug dosage and frequency.
Pharmacodynamics
Study of how drugs interact with the body to produce their effects. It focuses on drug-receptor interactions, drug concentration-response relationships, and the mechanisms of drug action.
Xenobiotics
Foreign substances, like drugs, chemicals, or pollutants, can harm living organisms and need to be detoxified or eliminated.
Blood brain barrier
A protective barrier in the brain that prevents harmful substances from entering the brain tissue, while allowing essential nutrients and oxygen to pass through.
Bioavailability (F)
The extent to which a drug or substance is absorbed and available for use by the body.
F = amount of drug that reaches circulation
amount of drug that is administered
prodrug
A medication that is inactive in its original form but is metabolized in the body to become an active drug.
Creatinine
A waste product produced by muscles during normal metabolism. It is filtered out of the blood by the kidneys and excreted in urine. Elevated levels can indicate kidney dysfunction or muscle damage.
P-glycoprotein
A protein found in the cells of the blood-brain barrier that acts as a pump to remove drugs and toxins from the brain, protecting it from potential harm.
Isozyme
Different forms of an enzyme that catalyze the same reaction but have different amino acid sequences.
Ligand
A molecule or ion that binds to a central metal atom or ion in a complex compound. It forms coordinate bonds with the metal, stabilizing the complex.
Antagonists
bind to specific receptors, blocking the actions of drugs or endogenous substances. They can counteract therapeutic effects or decrease overall activity.
Therapeutic Window
The range of drug concentrations in the body that produce the desired therapeutic effect while avoiding toxic side effects.
Therapeutic Index
Measure of a drug's safety margin. Calculated by dividing the lethal dose (LD50) by the effective dose (ED50). Higher value indicates a safer drug.
Semipermeable lipid bilayer
hydrophobic core presents major barrier
small, lipophilic molecules diffuse across
Carrier proteins
facilitate transport across the membrane
Blood-brain barrier (BBB)
endothelial tight junctions and astrocyte projections
Describe biological Barriers
Is the theoretical volume in which a drug would need to be uniformly distributed to account for its concentration in the body. It is calculated as;
the amount of drug in the body / plasma concentration.
Explain and calculate volume of distribution and clearance
Majority of drug metabolism occurs in liver
Cytochrome P450 enzymes (CYPs)
superfamily of enzymes 75% of drug metabolism
first pass effect
Drug interactions due to inhibition, induction, or competition
Renal elimination
filtration (passive)
secretion (active or passive)
reabsorption
considerations: pH of urine, weak acids and weak bases, effects of metabolism
Biliary elimination
size and lipophilicity/hydrophilicity
saturation
Describe metabolism-based drug interactions
Agonists: Activate receptors, mimicking endogenous ligands. Examples: Full agonists fully activate receptors, partial agonists partially activate, inverse agonists reduce basal activity.
Antagonists: Block receptors, preventing activation. Examples: Competitive antagonists compete with agonists for receptor binding, non-competitive antagonists bind to a different site, allosteric antagonists modulate receptor function indirectly.
Subtypes: Agonist subtypes include selective agonists (specific to certain receptors) and biased agonists (activate one pathway more than others). Antagonist subtypes include inverse agonists (reduce basal activity below normal) and silent antagonists (no effect on basal activity).
Define and explain in detail the pharmacodynamics of agonists and antagonists (and noted subtypes)
Affinity: The measure of how well a drug binds to its target receptor.
key parameter: KD
Efficacy: The ability of a drug to produce a desired effect once bound to its receptor.
key parameter: maximum response, relative to endogenous agonist
evaluated at saturating concentration, full occupancy
Potency: The concentration of a drug required to produce a specific effect. key parameter: EC50 or ED50
Explain affinity, efficacy, and potency
dissociation constant (Kd)
The measure of the strength of a chemical reaction between a ligand and a receptor. It represents the concentration of ligand needed to occupy half of the available receptor sites. A higher Kd indicates weaker binding, while a lower Kd indicates stronger binding.
solvated
refers to the process of a solute being surrounded and dispersed by a solvent, forming a solution.
entropy
Measure of disorder or randomness in a system. It increases with the number of possible arrangements. Indicates the amount of energy unavailable for useful work.
conformation
the specific shape adopted by a drug molecule that allows it to bind to its target receptor. It determines the drug's efficacy and affinity for the receptor, influencing its ability to produce a therapeutic response.
Irreversible binding
The term for the inability of a drug-receptor complex to dissociate. It occurs when a drug forms a strong covalent bond with its receptor, rendering the complex unable to separate. Non competitive
Pseudo Irreversible binding
Binding of a drug molecule to its target with high affinity and slow dissociation, leading to prolonged pharmacological effect. Non competitive
Stabilization energy
The energy required to maintain the binding of a drug to its receptor. It is crucial for drug efficacy and determines the strength of the drug-receptor interaction. It is influenced by factors like hydrogen bonding, electrostatic interactions, and hydrophobic interactions.
Induced fit
The concept where an enzyme changes its shape to fit the substrate more closely, enhancing the catalytic activity and forming an enzyme-substrate complex.
Drugs are solvated ligands that exist in conformational equilibrium mixtures
To form a drug-receptor complex, the drug must also displace solvent molecules from the binding site.
binding is difficult
must be in the correct conformation at moment of collision (in our example, less than 17% of collisions could lead to binding)
must displace solvent molecules
must overcome decrease in entropy
Describe the process of drug-receptor interactions
Noncovalent interactions for drug receptors include hydrogen bonding, van der Waals forces, electrostatic interactions, and hydrophobic interactions.
List and identify noncovalent interactions
Hydrogen Bonding
A “special” form of dipole-dipole interactions: Z-H (where Z = O, N, or F).
carries strong partial positive charge in this situation.
is usually stronger than other dipole-dipole interactions: provides approximately -1 to -7 kcal/mol stabilization (usual range -3 to -5)
binding affinities increase by about one order of magnitude per hydrogen bond
also is basis for DNA base pairing
Hydrophobic/ Van der Waals
interactions of temporary dipoles of hydrophobic, nonpolar groups (weakest interaction)
usually < 1 kcal/mol in strength
Charge Transfer complex (Special dipole-dipole interaction)
Intercalation interactions, similar in strength to dipole dipole
Shape and sterics are important for binding. Shape ensures a good fit between receptor and ligand, increasing binding affinity. Sterics affect atom arrangement, binding site accessibility, and binding strength
Describe the roles of shape and sterics in binding
Full agonist
A drug that fully activates a receptor, producing a maximum response. It has high affinity and efficacy, leading to strong biological effects.
99-100%
Partial agonist
A drug that binds to a receptor and has both agonistic and antagonistic effects. It activates the receptor, but to a lesser extent than a full agonist. 30-80%
Orthosteric agonist
A substance that binds to and activates a receptor site, causing a physiological response. Competitive
Allosteric agonist
A molecule that binds to a site on a receptor other than the active site, causing a change in the receptor's shape and enhancing the affinity and efficacy of the receptor for its endogenous ligand. Non competitive
Hormesis
Stress-induced response in which low doses of a harmful substance or condition can actually enhance an organism's resilience and health.
Dose response curve
Graph that shows the relationship between the dose of a drug or treatment and its effect on the body. It helps determine the optimal dose for desired outcomes and the potential for side effects.
Quantal concentration-response curve
shows the relationship between the concentration of a drug and the proportion of individuals affected.
Quantal dose-response curve
A graph that shows the relationship between the dose of a drug or toxin and the percentage of individuals affected. It helps determine the effective dose and toxic dose of a substance.
LD50
the lethal dose that kills 50% of the test population. It measures the toxicity of a substance.
Frequency distribution
A summary of data that shows how often each value or range of values occurs. It helps analyze patterns and identify the most common or rare occurrences in a dataset.
Physiological receptor
is a specialized protein molecule located on the surface of cells that detects and responds to specific chemical signals, such as hormones or neurotransmitters. It initiates a series of biochemical events, leading to a cellular response. Examples include G protein-coupled receptors and ligand-gated ion channels
Constitutive activity
Activation of a receptor in the absence of a ligand. It results in a basal level of signaling even without stimulation.
Endogenous ligand
A molecule produced naturally within the body that binds to a specific receptor to initiate a biological response
Hyperbolic
is a mathematical curve that resembles the shape of a saddle or two intersecting branches. It usually describes the relationship between binding ligand and concentration.
Inverse Agonist
A drug that binds to a receptor and produces the opposite effect of an agonist. It reduces the basal activity of the receptor below its normal level.
Allosteric potentiators
Compounds that enhance the activity of a receptor or enzyme by binding to a different site than the active site, causing a conformational change that increases the receptor's or enzyme's response to its natural ligand.
Qualitatively: Agonist binding activates the receptor, triggering a cellular response.
Quantitatively: Higher agonist concentration leads to more receptor binding and stronger cellular response.
Agonists bind to specific sites on receptors, such as orthosteric or allosteric sites, depending on the receptor type.
Explain qualitatively and quantitatively what happens when an agonist binds a receptor, and how/where agonists bind receptors.
Competitive antagonist: Binds to the same receptor site as the agonist, blocking its activity. decrease the potency of agonists
Noncompetitive antagonist: Binds to a different site on the receptor, altering its shape and preventing agonist binding.
Allosteric antagonist: Binds to a regulatory site on the receptor, modulating its activity. decrease potency and efficacy of agonist
Reversible antagonist: Can be easily displaced from the receptor, allowing agonist binding to resume.
Irreversible antagonist: Forms a covalent bond with the receptor, permanently inhibiting its activity. decrease potency and efficacy of agonist
Antagonists inhibit or reduce the activity of agonists, which are substances that activate receptors and produce a response.
allosteric potentiators increase the potency of agonists
List types of antagonists and describe their activity
Occupancy
The proportion of drug receptors that are bound by a drug molecule at any given time, expressed as a percentage. It determines the extent of drug-receptor interaction and influences the pharmacological response.
Fractional occupancy
measures the proportion of drug receptors occupied by a drug, indicating its effectiveness and potency. It is calculated by dividing the number of occupied receptors by the total number of receptors.
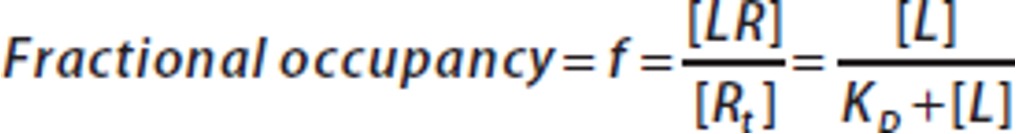
Spare receptors
Receptors that are not occupied by a drug, allowing for future binding. They can be targeted by additional drugs to enhance or prolong the desired effect.
Positive allosteric modulator
is a substance that enhances the activity of a receptor or enzyme by binding to a different site on the protein. It increases the response to the primary ligand without directly interacting with it.
Negative allosteric modulator
A molecule that binds to a receptor and reduces its activity. It causes a decrease in the response of the receptor to its agonist, leading to a decrease in the overall biological effect.
Potentiate
The process of enhancing the effects of a drug by combining it with another substance, resulting in a greater overall effect.
Downstream amplification
is the process where a small initial signal activates a series of intracellular events, resulting in a larger and more significant response.
Isobologram
Graphical representation used to determine if the combined effect of two drugs is additive, synergistic, or antagonistic.
Synergistic
When two or more drugs work together to produce a greater effect than each drug alone.
Additive
When two or more drugs are combined, and their effects are increased.
Negative synergism
is when two or more drugs have a combined effect that is less than the sum of their individual effects. This happens when the drugs interact in a way that counteracts each other, leading to a reduced therapeutic outcome.
Adverse Drug Reaction (ADR)
What is ADR?
Adverse Drug Event (ADE)
What is ADE?
Type A (ADR)
Drug-induced adverse reaction resulting from the pharmacological action of a medication. It is predictable, dose-dependent, and often related to the known pharmacological properties of the drug. Examples include nausea, drowsiness, and constipation. are more common and easier to manage than Type B (idiosyncratic) reactions.
Type B (ADR)
refers to unpredictable and idiosyncratic reactions to medications. These reactions are not dose-dependent and occur in a small subset of the population. They can involve various organ systems and are often unrelated to the pharmacological action of the drug. are typically not reproducible and may require discontinuation of the medication.
Institute for Safe Medical Practices (ISMP)
is a non-profit organization promoting safe medication practices. It offers resources, education, and tools to healthcare professionals to prevent errors and improve patient safety.
Confidential/ voluntary. For med/vaccine errors.
World Health Organization (WHO)
a global public health organization established in 1948. It operates as a specialized agency of the United Nations, aiming to promote health, prevent diseases, and improve healthcare worldwide. They set health standards, coordinates global health efforts, and plays a crucial role in addressing pandemics and emergencies.
Local case report form (LCR)
tolerance
The ability of an organism to endure or resist the effects of a drug over time, requiring higher doses to achieve the same therapeutic effect.
dependence
The state of reliance on a substance to function normally, leading to withdrawal symptoms upon discontinuation. It can be physical or psychological, with cravings and tolerance developing over time.
physiological and psychological
not the same as addiction
Addiction
A condition involving compulsive drug-seeking behavior and continued use despite negative consequences. It affects the brain's reward system, causing tolerance and withdrawal symptoms.
Tachyphylaxis
Rapid decrease in response to a drug or stimulus due to repeated exposure or administration.
Attenuation
Reduction of the intensity or strength of a drug's effect. It occurs when the drug's concentration decreases or when it is metabolized or eliminated from the body. Can lead to a decrease in therapeutic efficacy or the need for dose adjustments.
Desensitization
is the gradual reduction of sensitivity to a drug or allergen through repeated exposure. This can lead to decreased effectiveness of the drug or the need for higher doses. It can also lessen the severity of allergic reactions over time.
Downregulation
Decreased sensitivity of a cell to a drug due to prolonged exposure, resulting in a decrease in the number or affinity of drug receptors.
Sensitization
The process in which repeated exposure to a drug leads to an increased response over time. It occurs when the body becomes more sensitive to the effects of a drug, resulting in a stronger and more intense reaction. Can lead to enhanced drug effects and increased risk of adverse reactions.
AKA reverse tolerance
Up regulation
Increase in the number or sensitivity of receptors on a cell's surface in response to a drug or stimulus. Enhances the cell's response to the drug or stimulus, making it more effective. Can lead to increased cellular activity or production of a specific substance.
Supersensitivity
Exaggerated response to a drug due to increased sensitivity of target receptors. Can lead to excessive therapeutic effects or adverse reactions.
Biomarker
are measurable indicators used in assessing biological processes, drug response, and disease progression. They help diagnose diseases, monitor treatment efficacy, and predict patient outcomes. Can be proteins, genes, metabolites, or other molecules.
Factors contributing to variable drug response include genetic variations, age, drug interactions, lifestyle factors, disease status, gender, environmental factors, and ethnicity.
List factors that contribute to variable drug response
Pharmacophore
the portion of the molecule responsible for (and necessary for) pertinent receptor binding
Auxophore
the portion(s) of the molecule that are not essential for receptor binding
Hydrophilic/hydrophobic
1) Attracted to water, easily dissolves in water.
2) Repelled by water, does not dissolve in water.
Lipophilic/lipophobic
1) Having an affinity for fat or lipid molecules.
2) Having a fear or aversion to fat or lipid molecules.
Electronic Effects
Explains how functional groups impact electron distribution in a molecule, influencing reactivity. Functional groups can either donate or withdraw electrons. Donating groups stabilize positive charges, while withdrawing groups destabilize positive charges and stabilize negative charges.
Solubility effects
The ability of a functional group to dissolve in a given solvent. It depends on the polarity and size of the functional group. Polar groups like -OH and -COOH are soluble in polar solvents, while nonpolar groups like -CH3 are soluble in nonpolar solvents.
Steric effects
refers to the influence of bulky substituents on the reactivity and conformation of molecules. They can hinder or prevent certain reactions, alter reaction rates, and affect molecular shape.
Electronic effects, solubility effects, steric effects
What are the three things we must consider for every functional group?
Conformation Flexibility
The ability of a molecule to adopt different shapes or conformations due to rotations around single bonds, allowing it to adjust to its environment. a drug can be “locked” in its active state
resonance
sharing of electrons among atoms with conjugated pi (π) system (adjacent double/triple bonds)
Induction
effects of polarized (unequal sharing) sigma (σ) bonds (single bonds)
difference in electronegativity fluorine (F) > oxygen (O) > chlorine (Cl) > nitrogen (N)…
Phase 1 metabolism
The initial step of drug metabolism that involves chemical modifications, such as oxidation, reduction, or hydrolysis, to make the drug more polar and easier to eliminate from the body. Slow, CYP450, hydrolases, and other oxidative/reductive enzymes. Requires oxidizing agent (oxygen). Inactive, active, or toxic metabolites.
Phase 2 metabolism
The stage of drug metabolism where drugs are transformed into more polar compounds by conjugation with endogenous molecules like glucuronic acid, sulfate, or glutathione. This increases their water solubility, facilitating their excretion from the body. Fast, Conjugation of the phase I metabolite with an endogenous substrate to form a highly polar, ionized structure. Requires transferase enzymes. Requires a conjugate molecule. Usually inactive or nontoxic metabolites.
Oxidation
Process where a drug undergoes a chemical reaction with oxygen, resulting in the loss of electrons. increased polarity of covalent bond to create or increase δ+ charge. replacing a hydrogen or carbon on a drug molecule with oxygen. may form active or inactive metabolites-active metabolites may be toxic.
Reduction
Process of decreasing the amount or intensity of a drug's effects in the body, usually through metabolism or elimination.
Hydrolysis
Process in which a drug molecule is broken down into smaller components through the addition of water molecules. Esters and amides.
Conjugation
Attachment of hydrophilic molecules to drug molecules or metabolites by formation of covalent bond. Conjugate usually replaces a hydrogen covalently bonded to a heteroatom.
Conjugate
The process of linking a drug molecule to another molecule, such as a protein or antibody, to enhance its properties, such as stability, solubility, or targeting. can improve drug delivery, increase specificity, and reduce toxicity.
Ex. glucuronate, sulfate, amino acid (e.g. glycine), glutathione (GSH), acetyl, methyl
Glutathione
An antioxidant in our cells that protects against oxidative stress, supports immune function, and aids in detoxification. It is known as the 'master antioxidant' for its vital role in maintaining health. Tripeptide (Glu-Cys-Gly) found ubiquitously in mammalian tissue
Metabolism
Any chemical modification of xenobiotics
Usually decreases lipophilicity
Usually decreases or removes biological activity
Oxidation state is usually increased or unchanged
What are the 3 generalizations of metabolism?