Chemistry
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
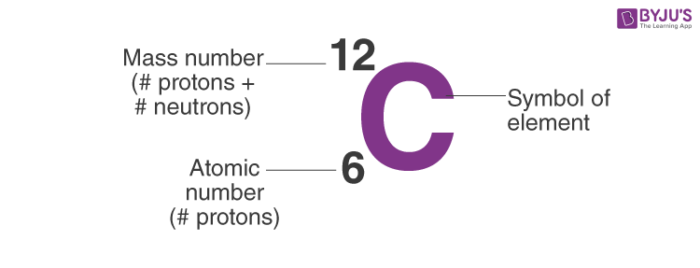
Atomic mass
The total mass of particles of matter in an atom.
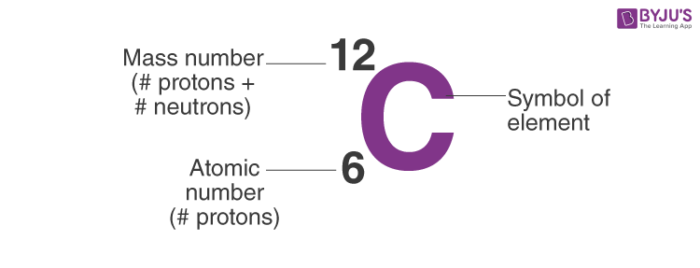
Atomic number
The number of protons in the nucleus of an atom, which determines the chemical properties of an element and its place in the periodic table
Nucleus
consist of electrically positive protons and electrically neutral neutrons.
Isopote
members of a family of an element that all have the same number of protons and electrons but different numbers of neutrons
The atomic number is determined by the number of...
Protons
Proton and neutron
The atomic mass is determined by adding the number of...
Electron
The particle that does not contribute to mass but determines the charge is the...
You determine an atom to be a negative ion. This means...
there are more electrons than protons.