Chemistry-Chapter 4: Stoichometry of Chemical Reactions
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
Rules for assessing oxidation states
1) Free elements have an oxidation state =0
2) Monoatomic ions have an oxidation state equal to their charge
3a) Sum of oxidation state of all atoms in a compound is 0. 3b) Sum of oxidation state of all atoms in a polyatomic ion equals charge of ion
4a) Group I metals have a +1 oxidation state in all compounds
4b) Group II metals have an oxidation state of +2 in all compounds
5) In their compounds, Nonmetals have oxidation states….
F -1 Group 7A (halogens) -1
H +1 Group 6A (chalcogens) -2
O -2 Group 5A (Nicogens) -3
Strong Acids
HCl H2SO4
HBr HClO3
HI HClO4 HNO3
Balancing Redox Reactions
1) Assign oxidation states
2)Write oxidation and reduction half reactions
3) Balance half-reactions by mass. a) balance elements other than H&O first b) Add H2O where O is needed. c)Add H+ where H is needed. *) If reaction is done in base, neutralize H+ with OH- 4)Balance half-reactions by charge. a) balance charge by adjusting electrons
5)Balance electrons between half reactions
6) Add half reactions
7) Count atoms and total charge to double check
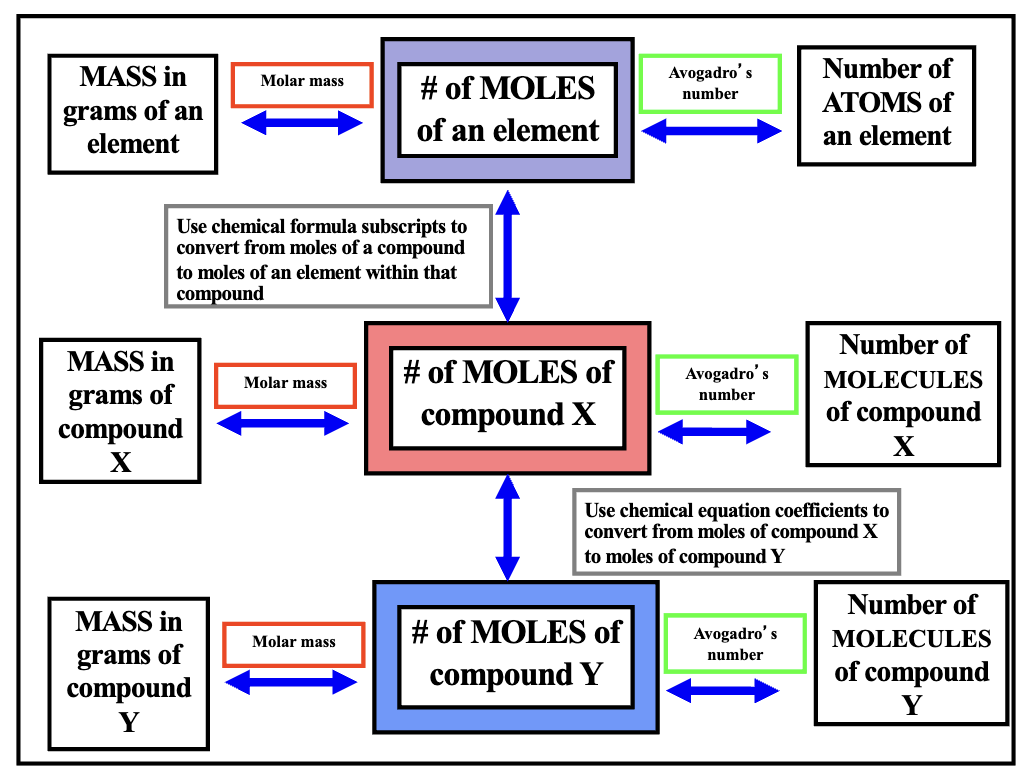
Conversion Factor Road map