module 2 water interactions and buffers
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
water is the most abundant molecule in living organisms. what is it’s passive and active roles?
passive; the structure of molecules forms in response to their interaction with water. (water not doing anything to the molecule just making it react to it)
active: water is a participant in many biochemical rxns for example a peptide bond releases a water molecule.
what non-covalent interactions are included in biomolecules?
1) hydrophobic interactions
2) electrostatic interactions
3) van der waals forces
4) ionic bonding
what do non covalent forces influence?
formation and stabilization of molecules, recognition, binding
describe how hydrogen bonding plays a role in structure and function of DNA
the structure of the dna nucleic acids will either be a donor or acceptor that will bond to the opposite of itself. this creates a structure with weak H-bonds that exist all throughout the molecule. these weak bonds however create stability when together. this affects function creating a very stable molecule yet an easily disassembled one for dna replication.
define hydrogen bonds
are electrostatic interactions between an electronegatibe atom with a hydrogen covalently linked( donor) to an atom with a free electron pair (acceptor)
describe waters ability to create H-bonds
every water molecule has two donors and two acceptors making it a very versatile molecule.
define ionic ( electrostatic interactions)
are interactions between a postive and a negative molecule either attracting or repelling.
the strength depends on the distance seperating the charges groups and intervening molecule.
define van der waals forces
interactions between permanent and induced dipoles. when two surfaces of complimentary shapes come together a large amount of atoms are brought into van der waals forces.
hydrophobic interactions
hydrophobic and hydrophiliic drive to have polar groups and non polar facing their respective compliments.
nonpolar side chains cluster in the interior of the protein.
describe how hydrophobic interactions contradict the second law of thermodynamics
although the folding of a polypeptide decreases entropy of the polypeptide it increases the entropy of water.
why does a slight change in pH cause drastic differences?
because pH is a log scale such that a 1 unit difference causes a 10x difference.
what does a titration curve tell us?
1)the ratio of acid to conjugate base over titration.
2) the buffer regions
3) the pKa and sequentially the pH
what does the buffer region tell us on a titration curve?
the region that resists changes by 1 unit on either side of the pKa.
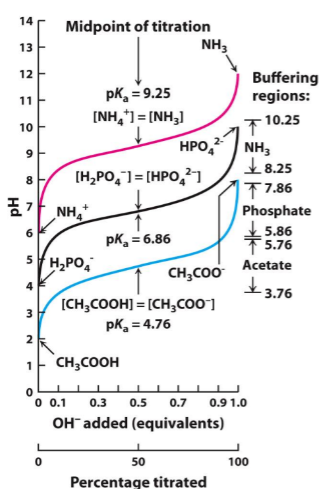
which one is the strongest acid?
Blue is the strongest acid
remember: Tthe lower the pKa the STRONGER the acid.
how can pH of a buffered sln be calculated?
can be calculated with the Henderson-Hasselbalch equation. pH = pKa + log [A-]/[HA] An equation relating the pH, the pKa and the ration of the concentrations of the proton accepts (A-) and proton donor (HA) species in a solution.
in cells and tissues what buffer systems maintain physiological pH?
phosphate and bicarbonate buffer systems in intracellular and extra cellular.
what is equal at pKa?
pH
what is an amphoteric molecule?
A molecule capable of donating and accepting protons, thus able to serve as an acid or a base.
what is the difference between intermolecular and intramolecular
intra: a process or characteristic within a molecule
inter: a process or characteristic within outside molecules.