chemistry: chapter 6 zumdhal 9th edition
0.0(0)
Card Sorting
1/34
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
1
New cards
q in
endothermic, positive q
2
New cards
q out
exothermic, negative q
3
New cards
work done on system by surroundings
contractive, positive w
4
New cards
work done by system on surroundings
expansive, negative w
5
New cards
heat
total energy of all particles
6
New cards
temperature
average speed of all particles
7
New cards
equilibria
heat content isn’t the same, but the temperature and heart flow is the same both ways
8
New cards
why is exothermic spontaneous?
in exothermic reactions, the products are more stable
9
New cards
exothermic reaction energy diagram
delta H is negative

10
New cards
endothermic reaction energy diagram
delta H is positive

11
New cards
if (w → 0)
delta E = q, proportional to delta T
12
New cards
if (q → 0)
delta E = w = F *d,* proportional to P \* delta V
13
New cards
melting and freezing
melting - expansion
freezing - contraction
Hfusion
freezing - contraction
Hfusion
14
New cards
boiling and condensation
boiling - expansion
condensation - contraction
Hvaporization
condensation - contraction
Hvaporization
15
New cards
sublimination and deposition
sublimination - expansion
deposition - contraction
deposition - contraction
16
New cards
state property
a propertiy in which we only care about the initial and final state, not the pathway in which we got there
17
New cards
calorimetry
study of heat transfer using a calorimeter
18
New cards
specific heat
the amount of heat it takes to increase one gram of a substance by one degree celcius
19
New cards
delta H =
\-qwater
20
New cards
bomb calorimeter is used when
there is lots of gas and high temperatures
21
New cards
qrxn =
\-qcalorimeter = -Ccalorimeter \* delta T
22
New cards
qsystem
qbomb+qH2O
23
New cards
phase change diagram
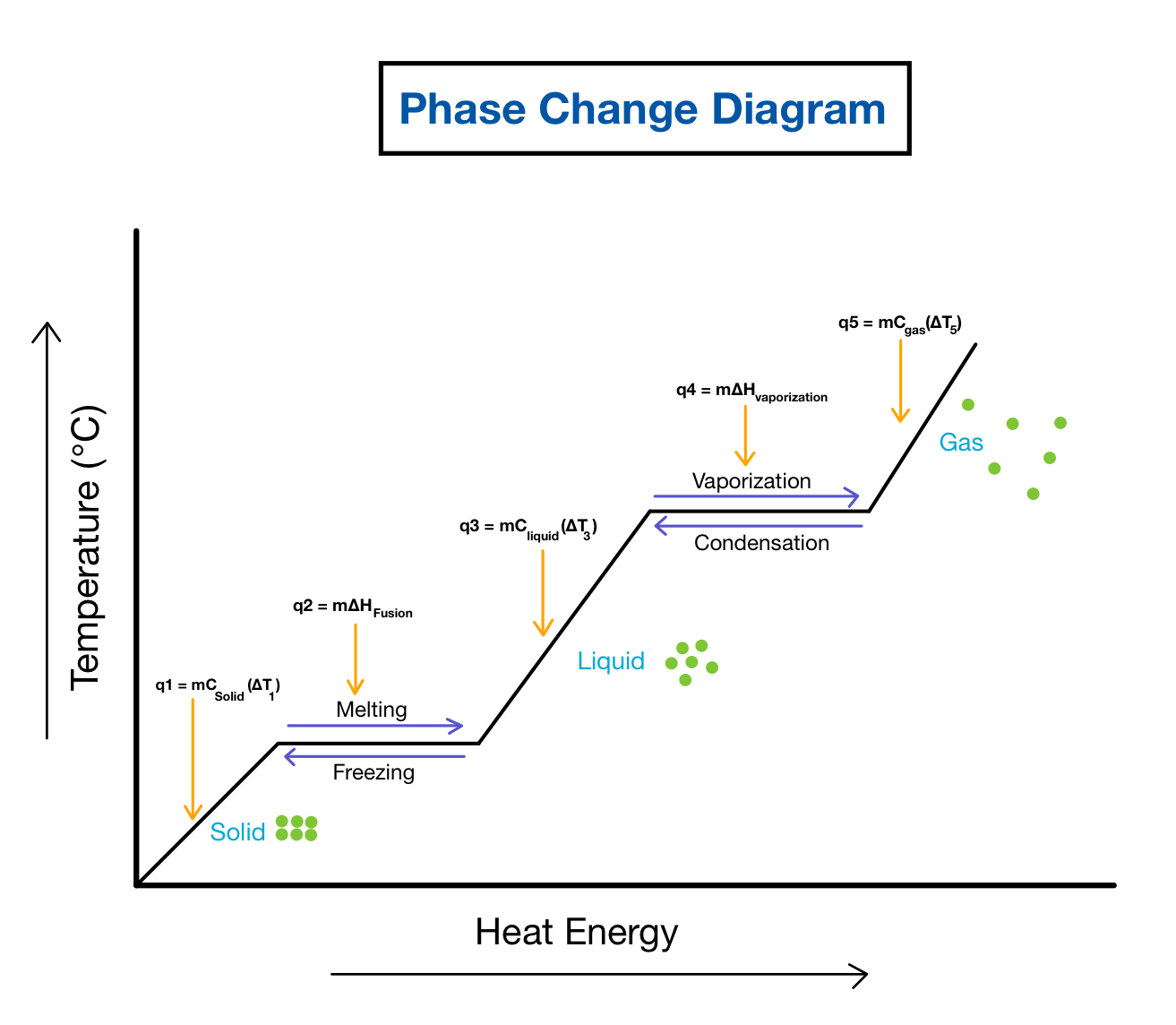
24
New cards
when the graph goes up
q = m \* c \* delta T
25
New cards
when the phase change graph stays constant
w = m \* Hx (x can be fusion or vaporization, it depends)
26
New cards
thermochem rule #1
delta H is directly propertional to the amount of reactands or products
27
New cards
thermochem rule #2
delta T for a reaction is equal in magnitude but opposite sign to delta H in reverse
28
New cards
thermochem rule #3
hess’ law
29
New cards
Hf
delta H when one molecule of stable compound is formed
30
New cards
bond energies
delta H when one mole of bonds are broken in the gaseous state
31
New cards
breaking bonds
positive
32
New cards
forming bonds
negative
33
New cards
lattice energy
energy released when two gaseous ions react to form a solid product
34
New cards
Hsolution
ionization energy + sphere of hydration
35
New cards
increasing lattice energy
least to most exothermic