(d) the periodic table
1/3
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
how are elements arranged in the periodic table (1.18 / 1.19)
elements are in order of atomic number (hydrogen’s atomic number is 1, helium’s is 2, lithium’s is 3 etc.)
groups (columns): elements in the same group have the same number of electrons in their outer shell i.e. elements in group 1 all have 1 electron in their outer shell etc.
periods (rows): elements in the same period have the same number of electron shells i.e. elements in period 3 all have 3 electron shells
how to classify elements as metals or non-metals (1.20 / 1.21)
metals conduct electricity, whereas non-metals do not; metals have oxides that are basic, whereas non-metals have oxides that are acidic
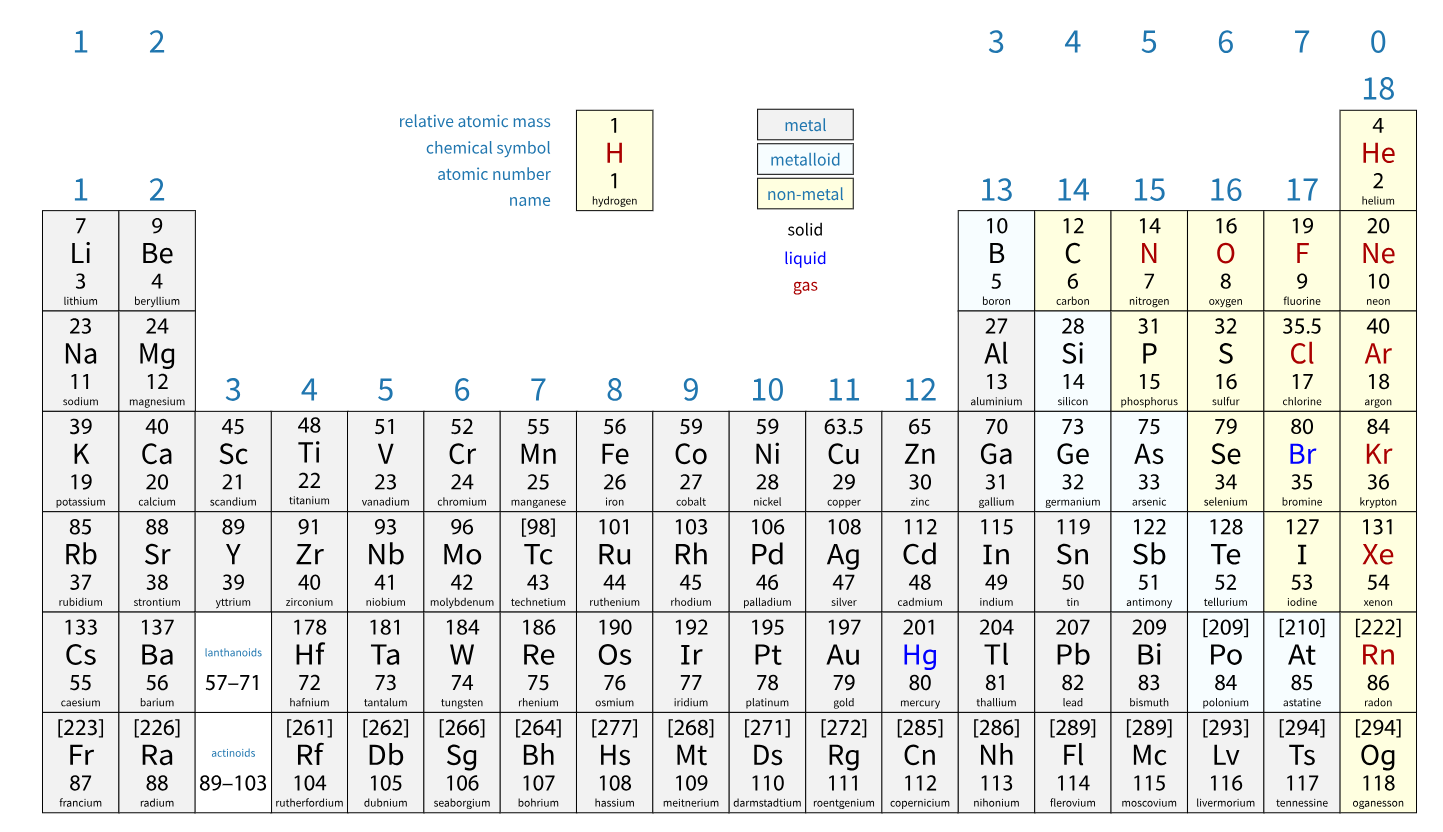
same group = similar chemical properties. why? (1.23)
when two atoms collide and react, it is the outer electrons that meet and interact. since elements in the same group have the same number of outer shell electrons, they have similar chemical properties.
noble gases (group 0) (1.24)
since atoms in group 0 (the noble gases) have a full outer shell of electrons, it is very unlikely that they will react with anything, since chemical reactions are usually caused by the outer electrons meeting and interacting. if the outer shell is full, the outer shell is stable and has no need to react with another atom to fill its outer shell.