Urine Cultures
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
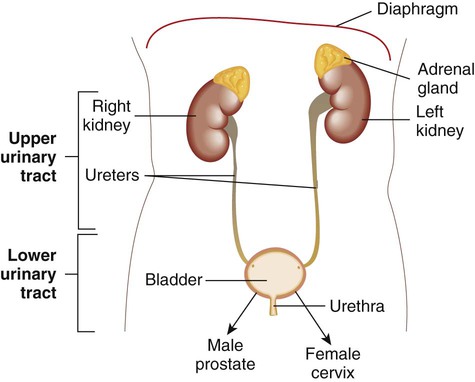
Urinary tract sterility
Urine is a sterile body fluid in healthy individuals
Upper tract: kidneys and ureters; lower tract: bladder and urethra
Resistant to colonization/infection due to:
Flushing action of urine
Mucosal immunity
Antimicrobial peptides
Presence of organisms may indicate:
Contamination (e.g. poor collection technique)
Colonization (e.g. catheterized patients)
True infection (e.g. ≥10⁵ CFU/mL with symptoms)
UTI epidemiology
UTIs are among the most common bacterial infections leading to doctor visits
Males: more common <1 year and >60 years (due to enlarged prostate)
Females: higher incidence due to shorter urethra and GI/sexual transmission
Risk increased by:
Diabetes
Renal disease
Structural abnormalities
Neurologic dysfunction affecting urine flow
UTI pathogenesis
Bacteria invade the urinary tract via two routes:
Ascending route — most common (e.g. entry through urethra)
Hematogenous/descending route — less common (e.g. seeding from bloodstream)
Host immune defenses typically eliminate invading organisms unless compromised
Types of urinary tract infections
Urethritis – inflammation of the urethra
Cystitis – inflammation of the bladder
Pyelonephritis (upper UTI) – infection of the kidney(s); more severe
Asymptomatic bacteriuria – presence of bacteria in urine without symptoms
Laboratory testing – UA with reflex to culture
Recommend urinalysis (UA) with reflex to culture when infection is suspected
UA findings that trigger reflex to culture:
Nitrites: positive (suggests presence of nitrate-reducing bacteria)
WBCs: pyuria (increased white cells)
Leukocyte esterase: positive (enzyme from WBCs)
RBCs may also be present
Culture is performed only if UA meets predefined criteria
Urine specimen types
Clean-catch midstream urine – most common outpatient method; minimizes contamination
Straight catheterized urine – reduces contamination; used in pediatric or non-ambulatory patients
Suprapubic bladder aspiration – sterile collection; used in infants or when contamination must be avoided
Indwelling catheter (foley cath) – collected from catheter port, not the bag; higher risk of colonization
Urine specimen transport
Collect in a sterile container
Refrigerate and transport within 24 hours
Boric acid transport tubes allow preservation unrefrigerated for up to 48 hours
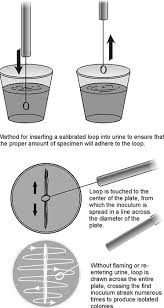
Urine culture setup
Thoroughly mix specimen before plating
Use calibrated loop (0.01 mL or 0.001 mL) for colony count
Streak for isolation and quantification
Common media (varies by lab):
5% sheep blood agar
MacConkey agar
CNA agar (for Gram-positive selection)
Chromagar (for differential ID)
Incubate at 35–37°C for 18–24 hours
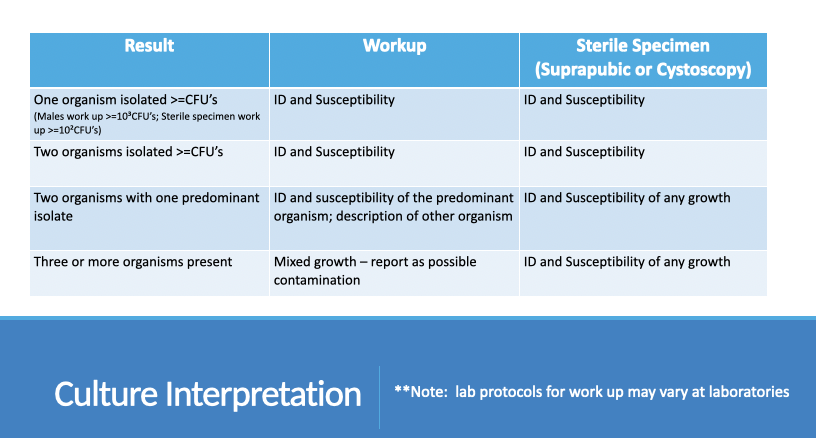
Urine culture interpretation
1 organism ≥ threshold CFU
ID and susceptibility
Threshold: ≥10⁵ CFU/mL (males), ≥10³ CFU/mL (sterile specimens)
2 organisms ≥ threshold CFU
ID and susceptibility of both
2 organisms with 1 predominant
ID and susceptibility of predominant
Describe secondary organism
≥3 organisms
Report as mixed flora
Suggest possible contamination
Sterile specimens (suprapubic/cystoscopy)
ID and susceptibility for any growth
Common urinary pathogens
Enterococci
Streptococcus agalactiae (Group B Strep)
Enterobacteriaceae (e.g., E. coli, Klebsiella, Proteus)
Pseudomonas spp.
Streptococcus pyogenes (Group A Strep)
Staphylococcus aureus
Staphylococcus saprophyticus
Candida spp.
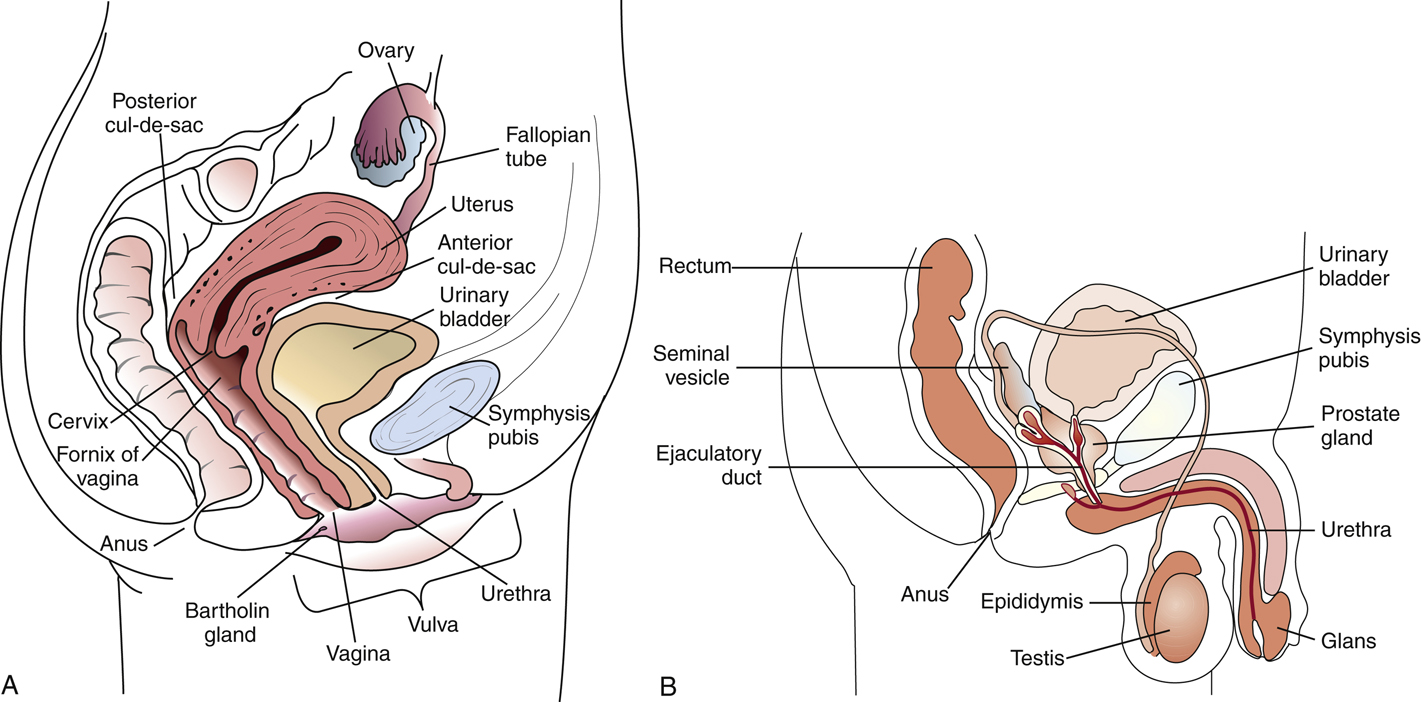
Anatomy and normal flora – genital tract
Female anatomy: proximity of urethra, vagina, and anus increases risk of contamination and ascending infections
Male anatomy: longer urethra and prostatic secretions offer some protection
Normal flora – females:
Lactobacillus spp. (predominant in healthy vaginal flora)
Corynebacterium, Staphylococcus, Streptococcus, Candida (low levels)
Normal flora – males:
Corynebacterium, Staphylococcus epidermidis, non-pathogenic Neisseria spp.
Normal flora must be considered to distinguish colonization from infection in genital cultures
Anaroebes
Normal genital flora
Coagulase-negative staphylococci
Corynebacterium spp.
Anaerobes
Lactobacillus spp. – predominant organism in healthy vagina
Streptococci
Enterobacteriaceae – more common in reproductive-age women
Genital infections and epidemiology
Some infections arise from endogenous genital flora due to:
Damage to protective barriers
Use of medical instrumentation
Introduction of foreign bodies
Most genital infections are sexually transmitted (STIs)
Genital conditions
Vaginitis
Inflammation/irritation of vaginal mucosa
Causes:
Bacterial vaginosis
Trichomonas vaginalis
Candida spp.
Symptoms: itching, burning, foul odor, dysuria, abdominal pain
Genital conditions
Cervicitis
Purulent discharge from endocervix
Common pathogens:
Neisseria gonorrhoeae
Chlamydia trachomatis
HSV
HPV
Genital conditions
Pelvic Inflammatory Disease (PID)
Complication of cervicitis
Most often caused by C. trachomatis or N. gonorrhoeae
Symptoms: vague and nonspecific; may include abdominal pain, discharge, fever, dyspareunia, dysuria, irregular bleeding
Genital conditions
Epididymitis
Inflammation of the male epididymis
Often a complication of Chlamydia trachomatis or Neisseria gonorrhoeae
Symptoms: scrotal inflammation, testicular pain/tenderness, dysuria, chills, fever
Genital conditions
Urethritis
Inflammation of the urethra
Symptoms: dysuria, urethral discharge
Gonococcal urethritis: Neisseria gonorrhoeae
Nongonococcal urethritis (NGU):
Chlamydia trachomatis
Less common: Ureaplasma urealyticum, Mycoplasma genitalium, Trichomonas vaginalis, HSV
Pathogens causing genital tract infections
Neisseria gonorrhoeae – urethritis, cervicitis, PID
Chlamydia trachomatis – urethritis, cervicitis, PID
Trichomonas vaginalis – vaginitis
Gardnerella vaginalis – vaginitis, clue cells
Treponema pallidum – syphilis
Haemophilus ducreyi – chancroid/lesion
Herpes simplex virus – herpes genitalis
Human papilloma virus – genital and anal warts
Candida spp. – vaginitis
Listeria monocytogenes – neonatal infection
Streptococcus agalactiae – neonatal infection
Genital specimen collection – culture setup
Cervix
Swab: moistened with Stuart’s or Amies media
Transport: within 24 hrs, room temp
Media: BAP, Choc, MAC, MTM
Genital specimen collection – culture setup
Urethra
Swab: Stuart’s or Amies media
Transport: within 24 hrs, room temp
Media: BAP, Choc, MAC, MTM
Genital specimen collection – culture setup
Vagina
Swab: Stuart’s, Amies, or JEMBEC (Thayer martin with pill that generates CO2 and then sealed in bag) system
Transport: within 24 hrs, room temp
Media: BAP, Choc, MAC, MTM
Can also perform Gram stain for bacterial vaginosis
Genital specimen collection – culture setup
Prostate
Swab (Stuart’s or Amies) or sterile container
Transport: swab within 24 hrs (RT); immediate if sterile container
Media: BAP, Choc, MAC, MTM, CNA, thioglycolate (thio)
Special considerations – genital culture collection
Use Dacron or Rayon swabs
Avoid calcium alginate swabs – toxic to some organisms
Gonococci isolation:
Transport in Modified Stuart’s or Amies charcoal media
If delay >12 hours, inoculate directly to culture media
Acceptable culture media:
Modified Thayer-Martin (MTM)
New York City (NYC) medium
JEMBEC plates (generate CO₂ atmosphere with tablet)
Genital smears
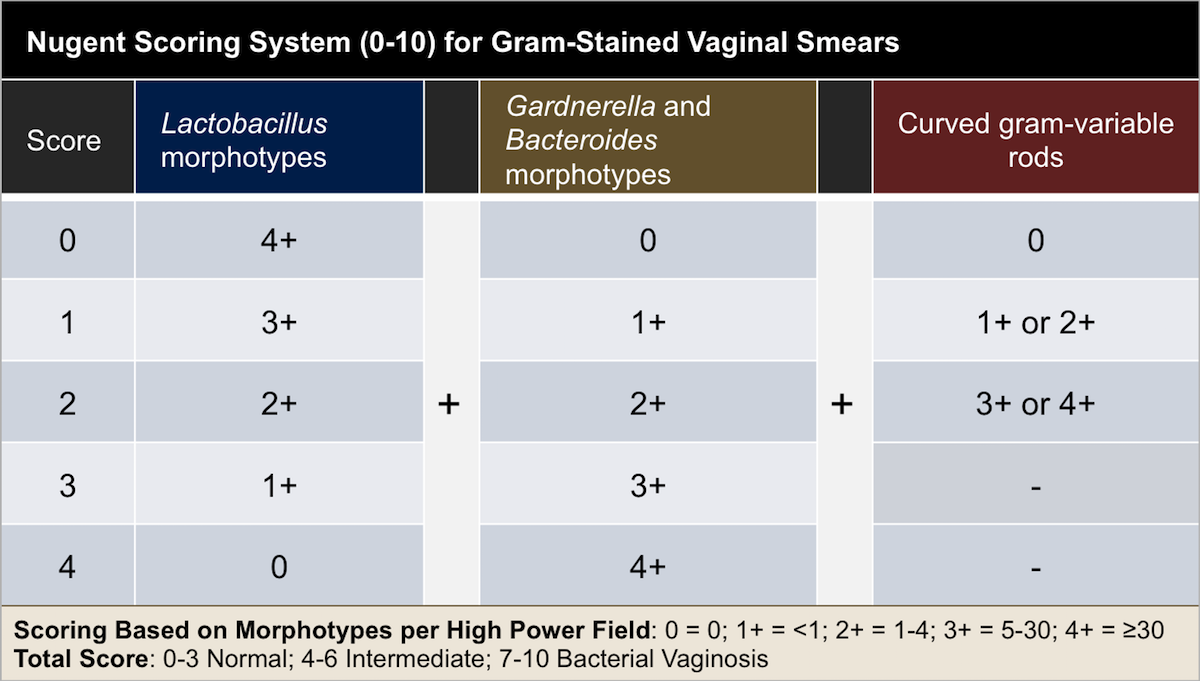
Gram stain
Detects Neisseria gonorrhoeae in urethral specimens (GNDC, intracellular)
Identifies clue cells in vaginal specimens (epithelial cells covered with bacteria)
Used in Nugent scoring system for diagnosing bacterial vaginosis
Genital smears
Wet mount/prep
Detects motile Trichomonas vaginalis
Must be performed promptly after collection to preserve organism viability