energy changes
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
what is an exothermic reaction and what are some examples
an exothermic reaction is one which transfers energy to the surroundings
some examples are combustion, neutralisation and hand warmers
what is an endothermic reaction and what are some examples
An endothermic reaction is one which takes in energy from the surroundings
Some examples are thermal decomposition and sport injury packs
how can you tell if it’s an endo or exo reaction
exo- there will be a rise in temp
endo- there will be a fall in temp
what is bendomexo
breaking bonds is an endothermic reaction
making bonds is an exothermic reaction
what is bond energy
the energy required to break a bond or the energy released when a bond is formed
what is activation energy
the minimum amount of energy needed before a collision will result in a reaction
how are chemical cells made
1)the 2 electrodes must be able to conduct electricity and so are usually 2 metals
2) the electrolyte is a liquid that contains ions that react with electrodes
3) the chemical reactions between the electrodes and the electrolyte set up a charge difference between the electrodes
4) of the electrodes are then connected by a wire the charge is able to flow and electricity is produced. A voltmeter can also be connected to measure the voltage of the cell
what does the voltage of the cell depend on
1) the type of electrodes used as diff metals react differently with the same electrolyte
2) the bigger the difference in reactivity the bigger the voltage of the cell(pd)
what is a battery
2 or more chemical cells connected in series
how can some cells be recharged
by applying an external current
why can some cells not be charged
the reaction can’t be reversed
what is a fuel cell
A cell that uses fuel and oxygen to generate electricity
what is the overall reaction in a hydrogen fuel cell
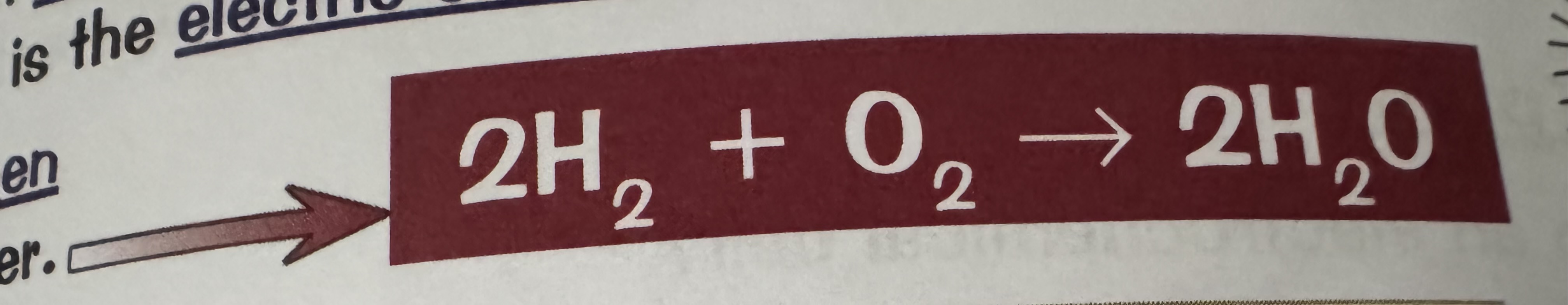
in an alkaline hydrogen fuel cell what are the half equations
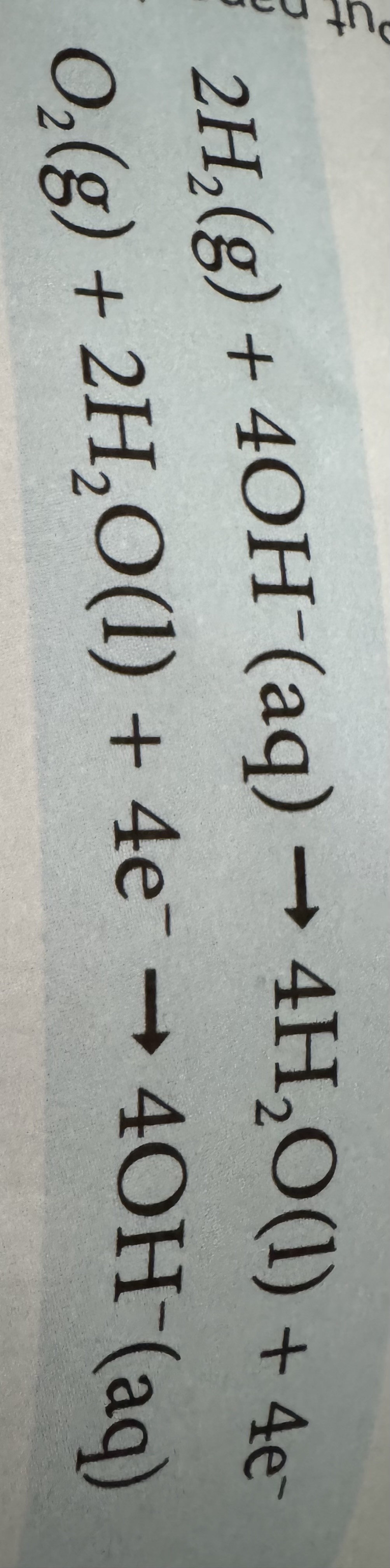
what are advantee of using hydrogen fuel cells
1) fuel cell vehicles don’t produce as many pollutants and other fuels
2) batteries store less energy than fuel cells and so would need to be recharged more often - which can take a long time
what are some disadvantages of using hydrogen fuel cells
Hydrogen is flammable s it’s hard to sore safely
2) hydrogen is a gas so it takes up more soace to store than a rechargeable battery