MOD MACULAR
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
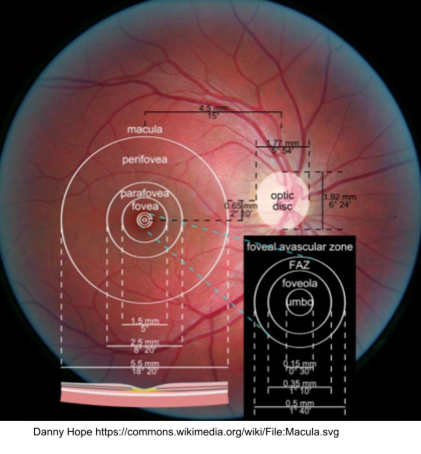
What is the approximate size of the macula and how much of total VF does it represent?
5.5mm in diameter - represents central 15-20 degrees
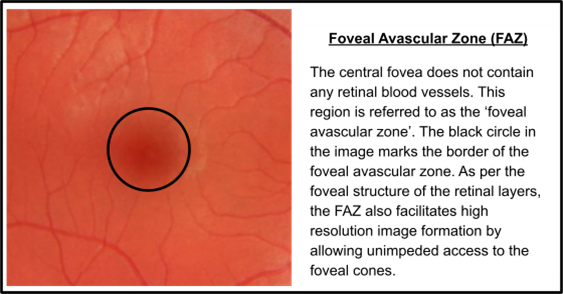
What is the fovea and its approximate size?
Shallow depression in the centre of the macula - critical for high resolution image (drives VA) despite occupying just 0.02% of the total retina - approx. 1.5mm in diameter - contains no BV referred to as the Foveal Avascular Zone
What are the percentages of cones and RGC at the fovea?
3% of cones total cones (200x greater cone density than the surrounding retina)
25% of total RGC
What is the foveola and what is its size?
Found at the centre of the macula - approx. 0.35mm in diameter
thinnest part of the retina
RGC are displaced laterally to minimise image degradation
What is the umbo?
Bottom of the foveolar pit and is the source of the reflex that is visible during fundoscopy
Absence of RGC but densely packed cones
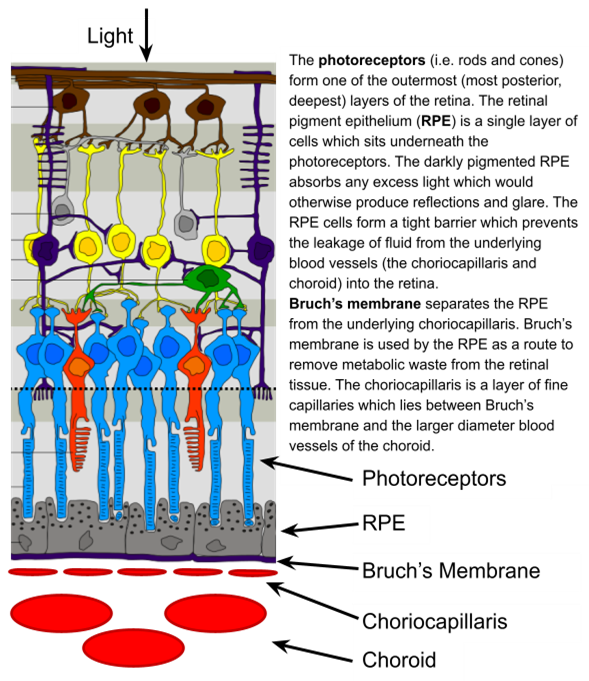
Functions of the RPE layer?
Absorbs any excess light which would otherwise cause glare and reflections
Form a tight barrier which prevents leakage of fluid from the choroid into the retina
Removes metabolic waste from retinal tissue via Bruch’s Membrane - 1 RPE cell collects debris from 30 cones
Provide nutrients to overlying cones via transport from the choroid - outer-blood-retinal layer
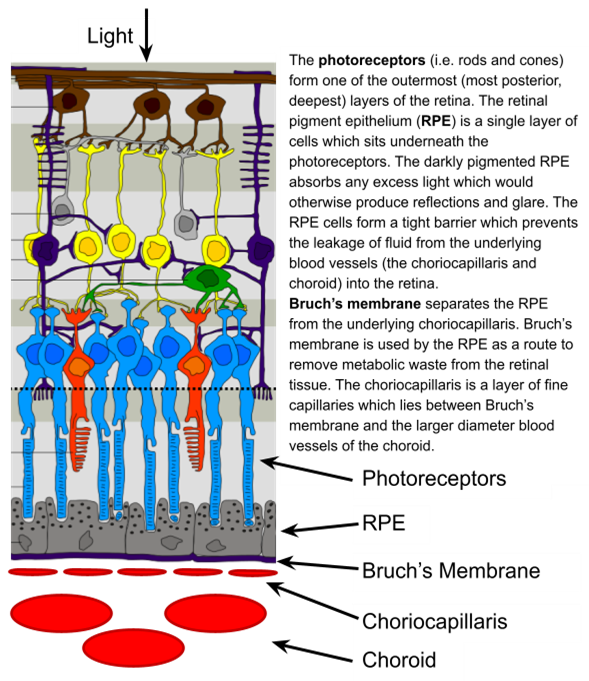
What is the function of Bruch's Membrane?
Key role in regulating the transportation of toxic metabolic waste from the retina into the choroidal blood circulation
When assessing the macula what tests should you carry out?
VA
Amsler chart
Retinal imaging and fundoscopy
Assessing VA, its link to macula disease + pinhole
Measure both Distance and Near, monocular and binocular
If macula disease, NEAR VA is often disproportionately impaired
Use the pinhole test - in px with compromised macula function VA will not improve as the factor limiting VA is non-optical (photoreceptors and RGC function)
The pinhole could make VA worse as there is a reduce FOV through the pinhole
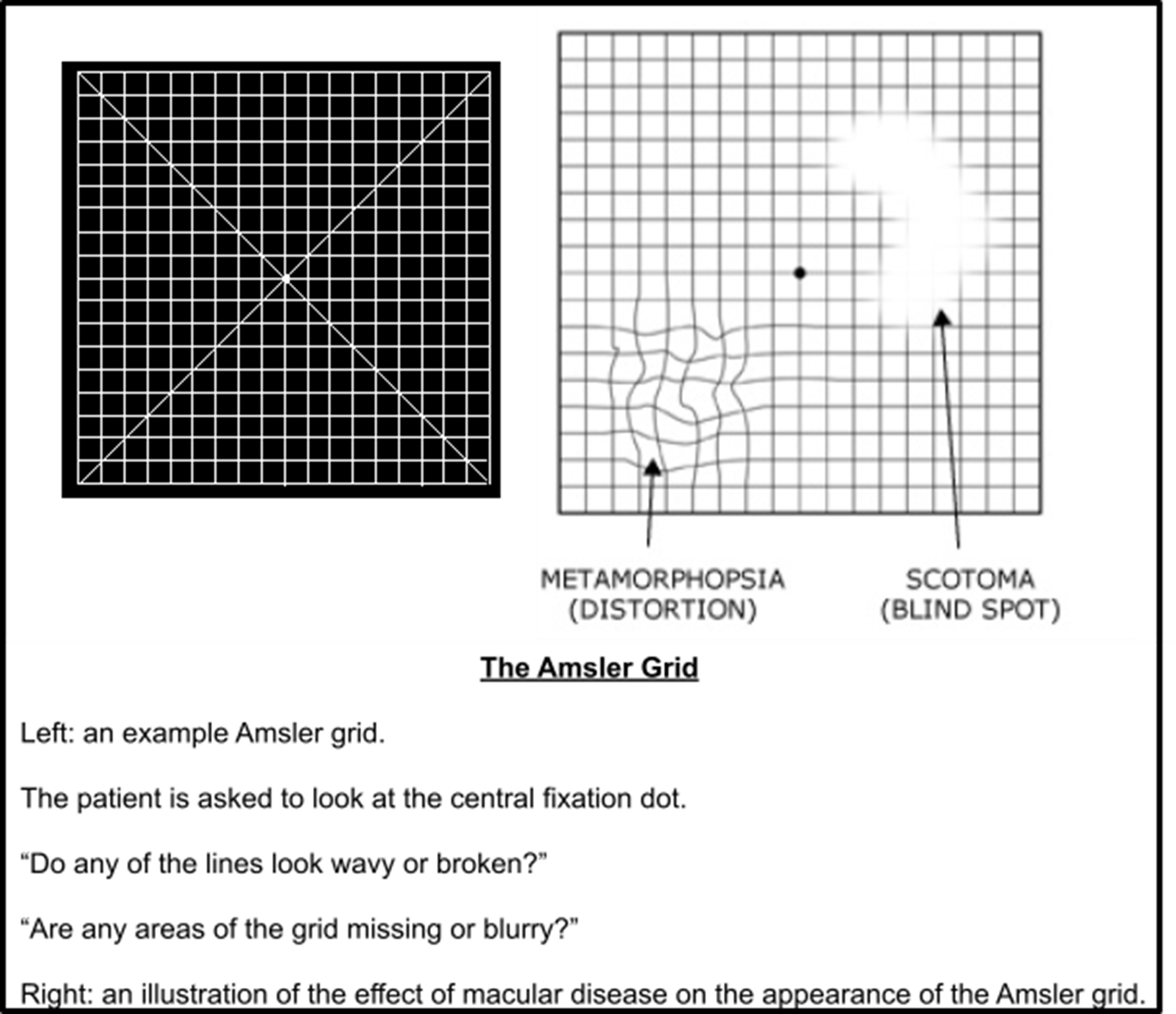
Amsler Chart
Sensitive assessment of the 20 degrees visual field
Test carried out monocularly at 30cm - each square subtends 1 degree of visual angle
Px need to wear correct reading Rx - NOT varifocals/bifocals
Px focuses on central dot and reports areas of distortion/scotoma
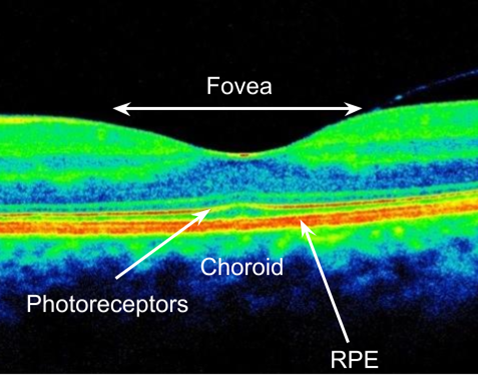
Retinal imaging/fundoscopy
Warrants a dilated fundus examination using Volk - allows detection of retinal oedema or elevation
OCT
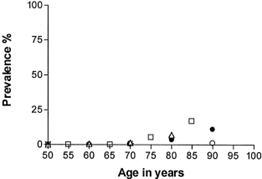
Prevalence of AMD in over 75s?, DRY/WET
Most common cause of irreversible visual impairment in the UK - increases exponentially with age
4.8% of over 65 have advanced AMD, rises to 12.2% of over 80s
30% of over 75s are in someway affected by AMD
Very rarely detected before 50
Dry AMD 90% of cases
WET 10% - typically more acute and severe - may require urgent referral
Is VA affected in the early stages of AMD?
Px may show visible signs associated with AMD but maintain good VA
Risk Factors for AMD
AGE - strongest RF
High BMI / History of cardiovascular disease / Hypertension - moderate association
Caucasians more likely than Africans or Asians
First degree relative with AMD - 3x more likely
Females
Smoking - Single most powerful modifiable RF - 2x the risk of developing AMD
What protects a px from AMD?
Healthy diet, regular exercise, normal BP and controlled cholesterol levels
How will AMD appear?
Bilateral, often asymmetric
Px with advanced AMD in one eye - 50% chance of advanced AMD in the fellow eye within 5 years
What are the terms used for AMD that describe the development of geographic atrophy?
Dry
Non-exudative
Atrophic
What are the terms used for AMD that are characterised by neovascularization?
Wet
Exudative
Neovascular
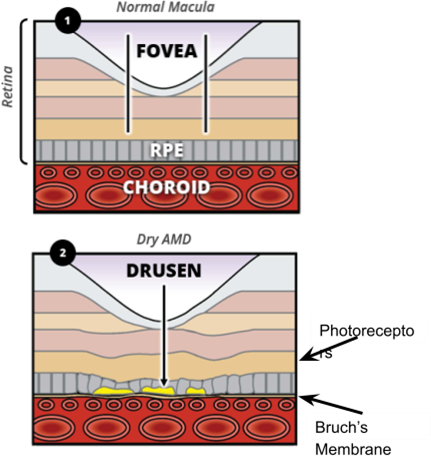
Why and How does AMD occur?
Ageing - causes an increase in thickness of Bruch’s membrane which reduces the permeability
This inhibits the removal of toxic metabolic waste such as Lipofuscin
These waste products begin to accumulate between Bruch’s membrane and the RPE (drusen)
SX of AMD
Bilateral - asymmetric and likely to be asymptomatic in the early stages
Gradual deterioration of vision - over a number of years
Advanced AMD - difficulty with visual tasks that require resolution of fine detail e.g reading/recognising faces
In severe cases a positive central scotoma
SX exclusive to WET AMD
Painless and sudden onset of blurred or distorted central vision - requires urgent referral - even if no retinal signs
Initial onset in Px with WET AMD is unilateral BUT 37% develop in 2nd eye within one year
Vision becomes distorted - metamorphopsia
Why does Metamorphopsia occur?
Due to the disruption of the organisation and orientation of the RPE and PRC by subretinal fluid
Signs of AMD
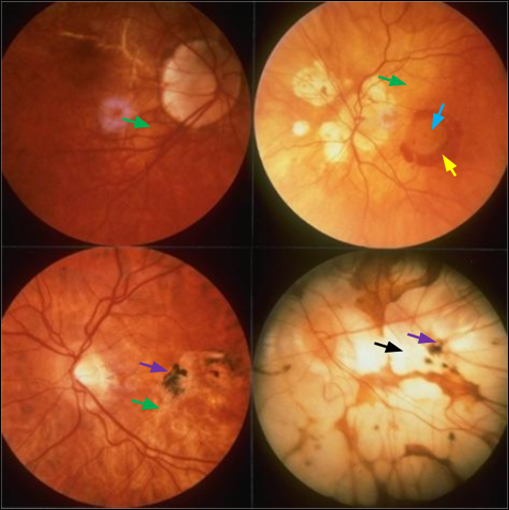
Drusen - first visible sign in both forms - typically clustered around the macula
Geographic Atrophy - DRY AMD + WET AMD
Choroidal NV - WET AMD
Haemorrhages - WET AMD

Summary of Drusen
Made up of waste products such as lipids and collagen due to build up between RPE and Bruch’s membrane - Cones have exceptionally high metabolic activity = large amount of waste products
Drusen disrupt the orientation and organisation of RPE cells - end result leading to atrophy
Drusen associated with depigmentation and hyperpigmentation
What do Drusen indicate?
They’re a common finding in over 50s so don’t necessarily indicate the presence of AMD!
Positive association between the number + size of drusen and the severity of AMD
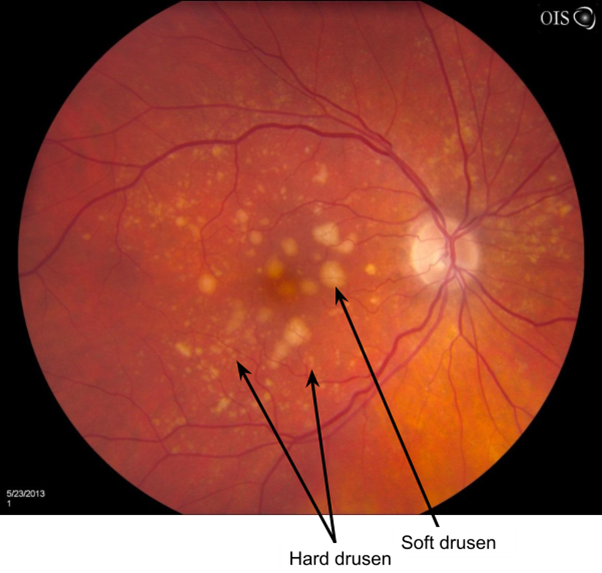
Hard Drusen
Small, yellow, well-defined with sharp edges - carry a low risk of visual impairment
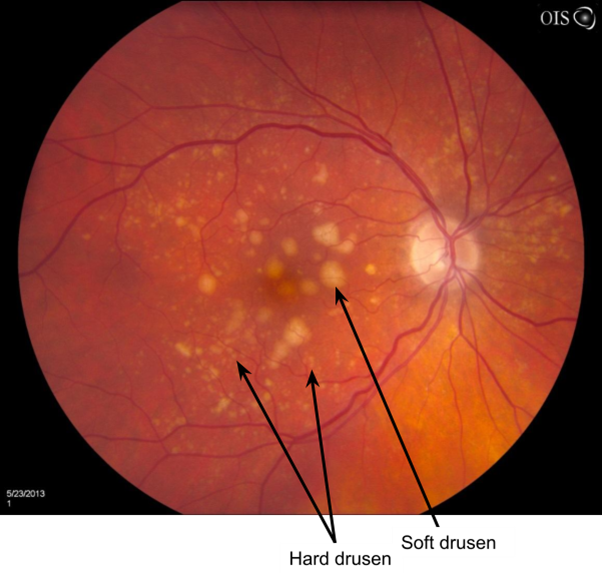
Soft Drusen
Larger, poorly defined fully edges - greater potential to disrupt the structure of the overlying RPE
Confluent - aggregate together to form a single area of drusen
Greater risk of visual impairment
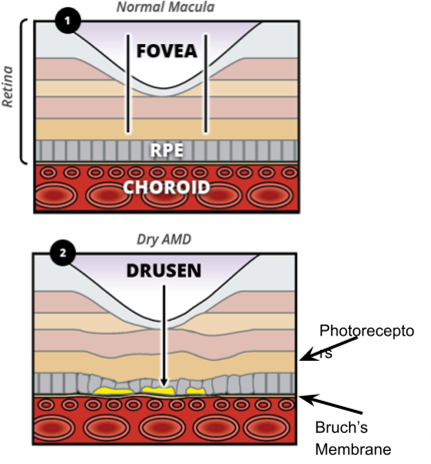
What does Drusen do to the retinal layers?
Elevates the RPE and distorts the structure+organisation of overlying retinal layers
Due to the above = reduce VA
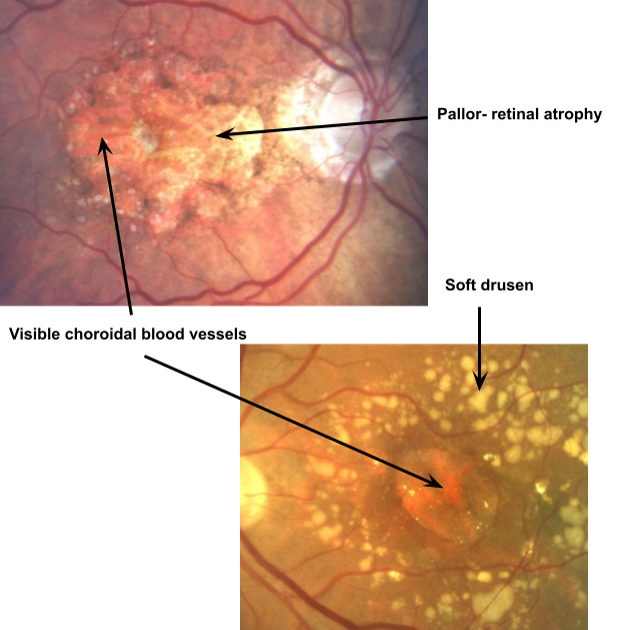
Geographic Atrophy
Degeneration of RPE leads to ATROPHY - loss of sensory retina = devastating impact on VA especially if the fovea is affected
END stage of DRY AMD
Px can develop a positive central scotoma in the region of the GA
Areas of GA can be identified by areas of retinal pallor
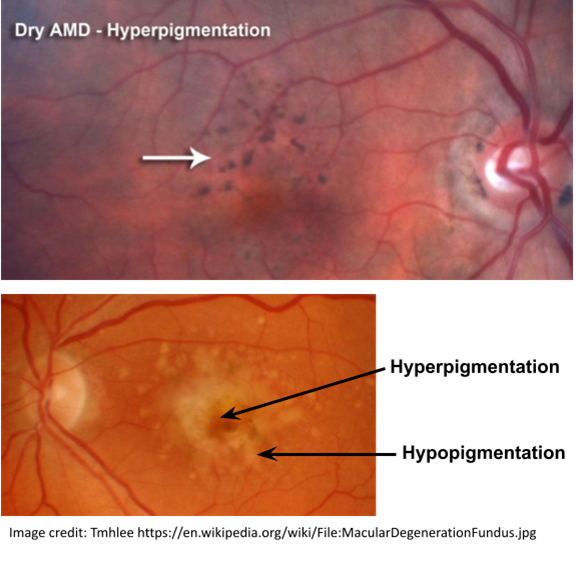
Signs of RPE degeneration?
Patches of both hypo- (brighter) and hyper- (darker) pigmentation - ‘stippling’ effect
What does GA do to the retina?
Reduces thickness of retinal tissue and exposes underlying BV of choroid and choriocapillaris
What is retinal pallor surrounded by and why does it arise?
Dense drusen
Hyper- and hypo- pigmentation - ‘pigment clumping’
Occurs due to lack of incident light absorption by the absent RPE cells
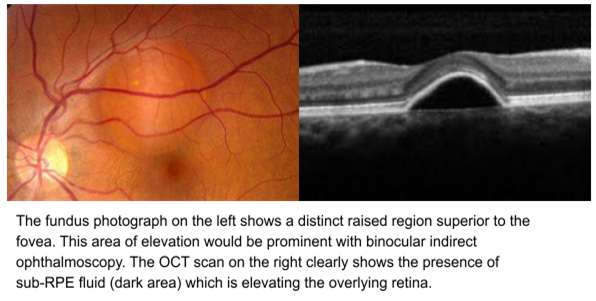
Choroidal Neovascularization
Hallmark for WET AMD
10-15% of px with Dry will develop WET AMD - Px with soft drusen and GA are at risk of CNV
It is possible for CNV without any signs of Dry AMD but very uncommon
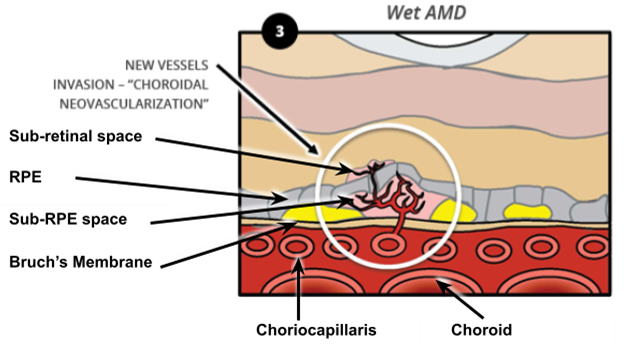
Why and How does CNV occur?
The angiogenic stimulus (trigger for CNV to occur) is ischaemia
Reduction in permeability of Bruch's Membrane = undersupply of oxygen to retina = new vessels arise from Choroid
This is supported by the proliferation of the subretinal neovascular membrane which is the tissue underneath the retina - this membrane will appear grey-green-yellow in colour - only in WET
This extends vessels of the choroid through defects in Bruch’s membrane to the sub-RPE space
Some vessels breaks through the RPE and grow into the sub-Retinal space
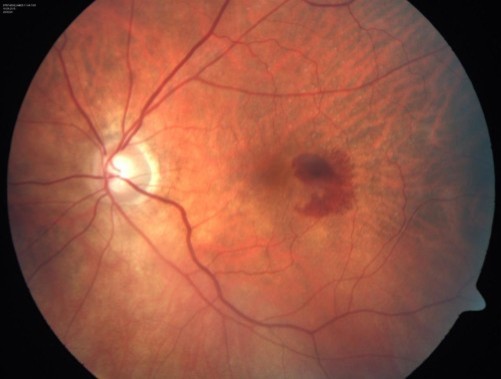
What occurs as a result of CNV
Leakage of blood = haemorrhaging within the macula region
Darker blood = deeper haemorrhages = sub-RPE - due to light absorbing properties of the RPE
Macula Oedema - driven from exudation of Choroidal BV, not retinal BV which is the case in DR, HTR and post-operative CMO - Sign of ONGOING exudation in AMD
Oedema is centred on the macula region and may be visible with volk as retinal thickening
Why does Oedema occur?
‘macromolecules‘ leaking from BV via an oncotic pressure gradient draws the water component of blood out of the vessel
Over time some of that aqueous content is reabsorbed by other vessels OR pumped into the choroid by the RPE
However the Lipoprotein component of the exudated fluid isn’t reabsorbed and left in the form as hard exudates
What are hard exudates a sign of?
Some exudated fluid has be reabsorbed but don’t necessarily signify an end to exudation
Usually occur in the mid retinal layers rather than sub-retinal space
Management for CNV?
Urgent referral even when haemorrhaging isn’t present as prognosis becomes poor once haemorrhage occurs
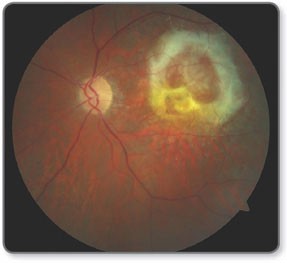
What is the end stage of exudative AMD?
Disciform scar - circular scar around the macular region
Caused by the proliferation of fibrous tissue which is produced to support CNV
If extends across fovea = very poor VA e.g hand movements - further vision loss is unlikely
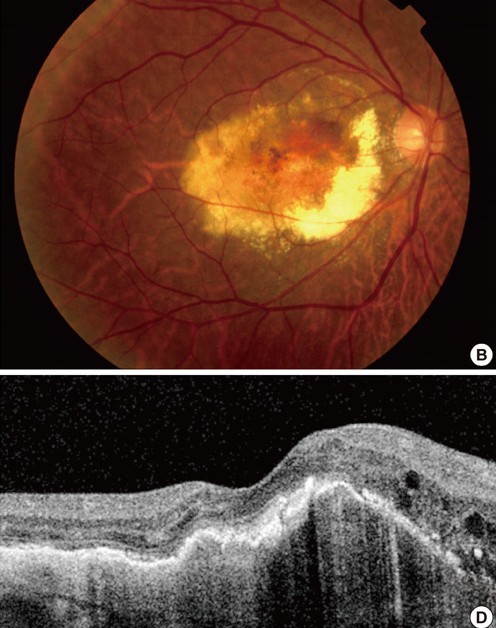
What does a Disciform scar look like?
Pale white/yellow circular-ish region with relatively well defined margins
Sub-retinal haemorrhage and oedema may be visible within the scar
Its surface tends to be irregular and will have differences in elevation throughout the scar
Small number of drusen is classified as?
‘Normal’ no AMD
AMD classification within NICE framework
DRY AMD - drusen = Early AMD
AMD - subretinal fluid = ‘Late AMD (indeterminate)’
DRY AMD - GA = Late AMD DRY
WET AMD - CNV = Late AMD (wet active)
WET AMD - Disciform Scar = Late AMD (wet inactive)
Optometric Management of Dry AMD
If optometrist confident about diagnosis, px doesn’t require referral but made aware of sx - annual recall
If not sure about diagnosis of Dry AMD - refer
Refer if px is symptomatic as likely to need reassurance about their vision loss
VA can become severely impaired LVA assessment may be indicated - refer via GP
Explain that AMD is a progressive condition and vision might start to deteriorate however the rate of progression is very slow - there is variability in different px in the rate of progression
Explain it will only impair central vision - they will still be able to navigate their environment
Explain that it is possible to develop WET AMD at any stage of DRY AMD - make aware of sx of WET - sudden reduction in vision or metamorphopsia
Amsler chart for self-monitoring
Ask px to regularly compare vision in the LE and RE whilst looking at a regular straight line target
Explain likely to be some deterioration in vision but isn’t a forgone conclusion - progression may be so slow
Make aware of modifiable RF - STOP SMOKING - will be beneficial even after AMD has been diagnosed
What should a px with AMD do if they feel their vision is deteriorating?
Seek an eye exam - sx are gradual in both WET?? and DRY but CAN be sudden in WET
Optom can then decide on referral
What are antioxidants?
Antioxidants have a protective effect on retinal cells
Combat action of free radicals which are produced by oxidative damage associated with incidence of light on the photoreceptors

What role does diet play in the development of AMD?
Macula has an abundant source of harmful free radicals - increases with AGE
Macula contains lutein and zeaxanthin - known as carotenoids - have antioxidant properties - causes yellow pigment
This pigment absorbs harmful short wavelength(blue) which would otherwise damage the retina - can’t be produced by the body so must be extracted from our diet
Leafy green vegetables - e.g spinach - good source of carotenoids - can also take supplements such as Viteyes
Increasing the proportion of antioxidants enhances the retinas ability to withstand harmful effects of free radicals
This may lead to the control of progression of AMD - does NOT delay or prevent onset of AMD
Vitamin C, E, and the carotenoids + lutein/zeaxanthin
Make px aware diet only plays a small role in the clinical procedure of AMD
What would you recommend to dispense AMD px?
UV protection coated lenses can reduce progression of AMD
Optometric Management of WET AMD
Urgent referral to Ophthalmologist using the Wet AMD ‘fast track’ pathway - aim to be seen within 2/52
Email the completed referral letter to a dedicated macula clinic - bypasses GP and reduces waiting time
If unsure of whether Dry/Wet still use the fast track pathway
If no fast track pathway available then same day phone call to HES
Last resort be referred to A&E
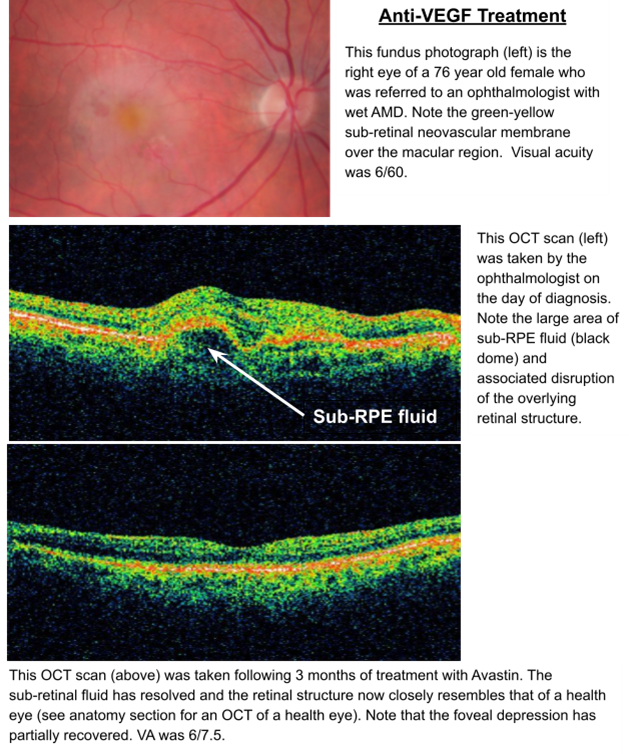
Ophthalmological management of WET AMD
Intravitreal injection of anti-VEGF agents - block action of VEGF - occur due to Ischaemia
Examples include; Ranibizumab (Lucentis), Bevacizumab (Avastin), Aflibercept (Eylea)
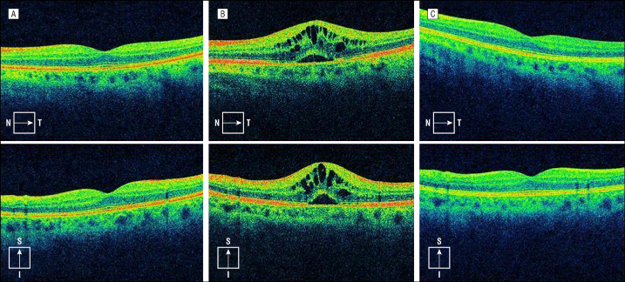
Cystoid Macular Oedema
Breakdown of inner BRB - pathological response to some form of injury, inflammation or vascular disease
Leakage of intravascular contents from dilated perifoveal retinal capillaries causes thickening of macula - may reduce macula reflex
Intraretinal fluid - leads to a flower-petal arrangement - displaces the GC layer
Leakage occurs within the INL AND OPL of the retina
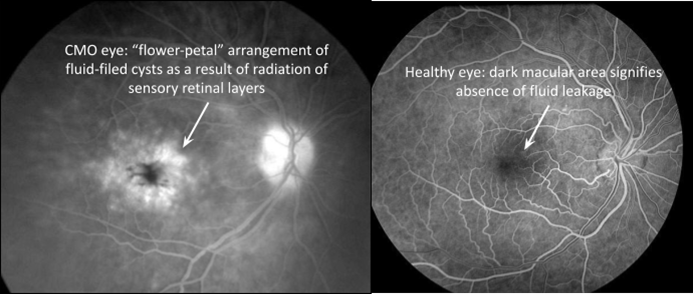
What are the causes of CMO?
Cataract extraction - 20% of cataract surgery px show some form of CMO - only 1% show significant reduction in VA
Most common cause of unexplained vision loss after surgery - can be termed ‘Irvine-Gass syndrome’ if occurs after cataract extraction
Other causes include uveitis - posteriorly more common, DR and CRVO/CRAO
Sx of CMO
Sudden, painless onset of blurred or distorted central vision
Develops over the course of 1-2 weeks
Many px with subtle CMO will be asymptomatic
Optometric Management of CMO
Same day phone call with Ophthalmologist
In cataract induced CMO there will be little to no retinal signs
If suspicion of uveitis then emergency referral
If suspicion CRVO/CRAO call HES for advise
Ophthalmological management of CMO
First line treatment = treat underlying cause e.g stabilise blood glucose in DM
Carbonic Anhydrase Inhibitors - increase fluid outflow via the RPE
Corticosteroids - reduce inflammation
Laser Photocoagulation
Increasingly treated with anti-VEGF drugs - reduce vascular permeability
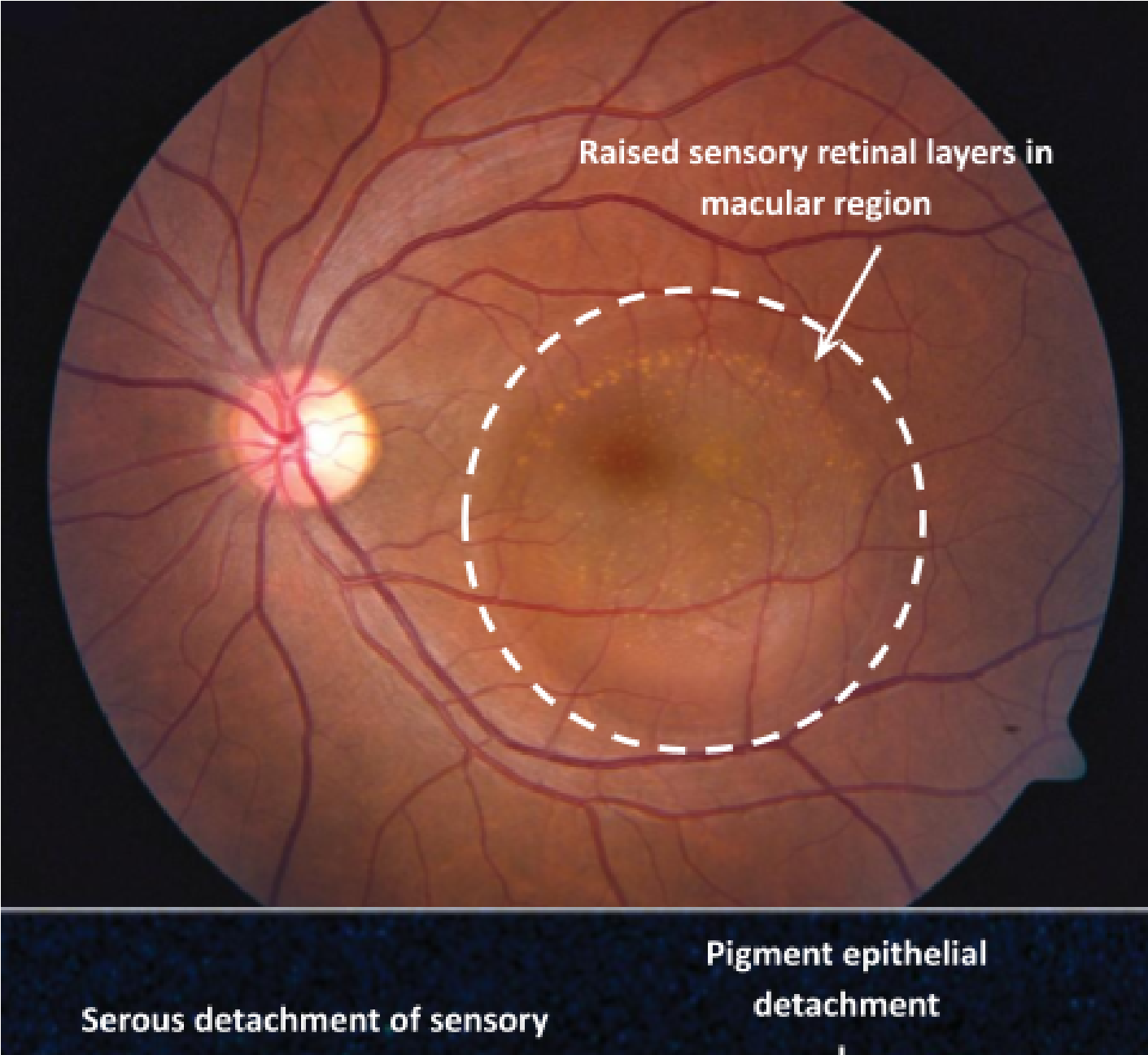
Central Serous Retinopathy
Arises due to ingress of fluid from the choroid and breakdown of the outer BRB
Irregularities of the RPE allow passage of serous fluid from the choroidal space into the sub-RPE space and subsequently into the sub-retinal space
Risk factors for CSR
Mostly within 20-50 age bracket
Males - who are more prone to stress/anxiety - cortisol (hormone) causes changes in choroids structure and function
Typically unilateral
30% of cases are recurrent episodes
Sx of CSR
Sudden, painless onset of blurred or distorted vision
Signs of CSR
Serous elevation of macula region (reduces axial length) and reduced VA
Potentially a positive shift in Rx
Management of CSR
70% of cases resolve spontaneously with good recovery of VA
Ophthalmological opinion is required to confirm diagnosis and rule out CNV
If confident of diagnosis refer px to HES to be seen within 4/52 - tell px if sx worsen return to Optometrist
If in doubt then seek opinion from local AMD fast track or call HES - URGENT
How to differentiate between CSR and CNV?
Px’s age - younger in CSR
Asking about px recent stress/anxiety levels
OCT will show absence of AMD features such as drusen
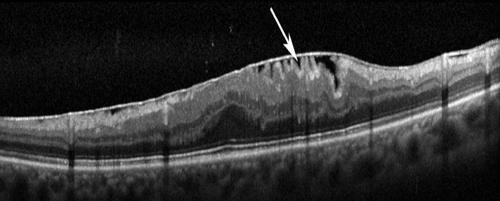
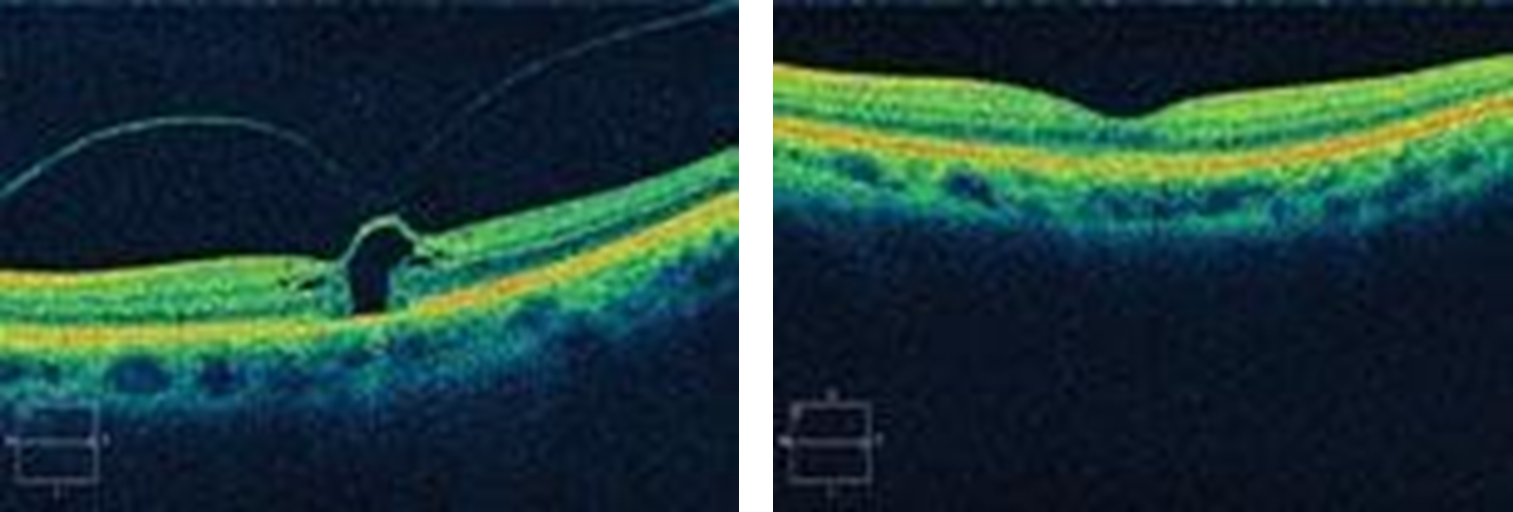
Epiretinal Membranes (ERM)
Disruption of ILM causes Muller cells + other cells to proliferate = epiretinal membrane
Can occur anywhere but most commonly the macular region
ERM are prone to contraction which causes retinal layers to become distorted - ‘bunched up’ appearance on OCT
Causes include; tractional forces during PVD, posterior uveitis (inflammatory process)
Most are idiopathic
Slow progression - take at least a year to develop
Sx of ERM
Reduced VA, metamorphopsia in severe cases (25%)
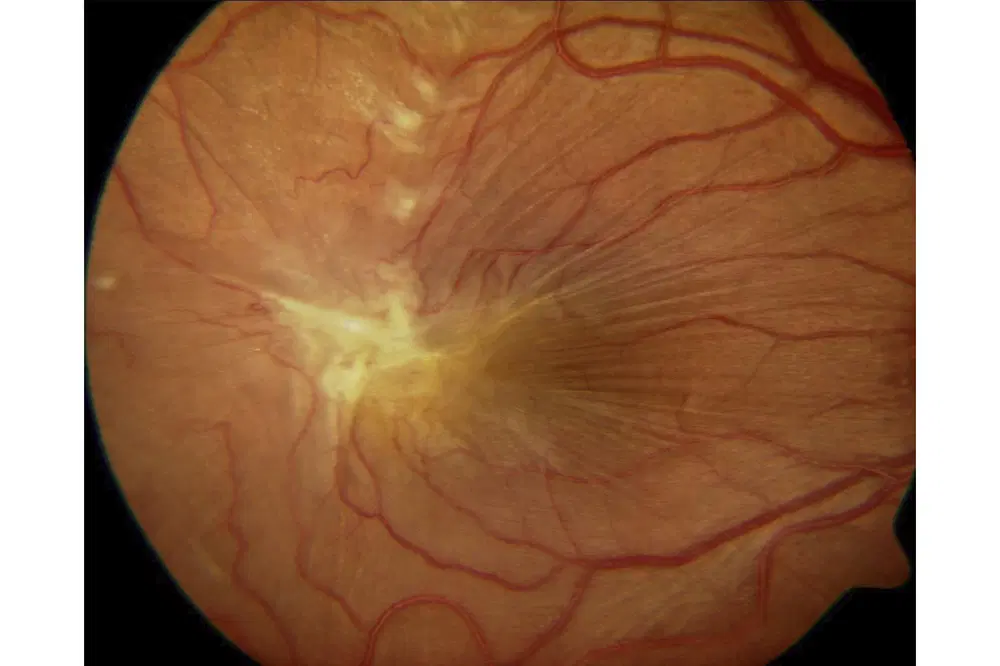
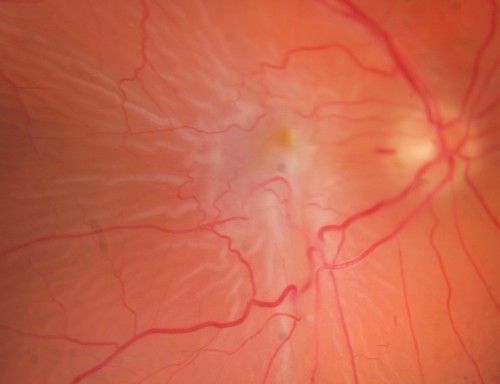
Signs of ERM
Early stage - Reflective, shimmering area around the macula - like the macula of a young px but onset at least 60 years old - referred to as cellophane maculopathy
VA will be unaffected or limited to a 1 or 2 line drop due to the ‘shiny’ retinal surface
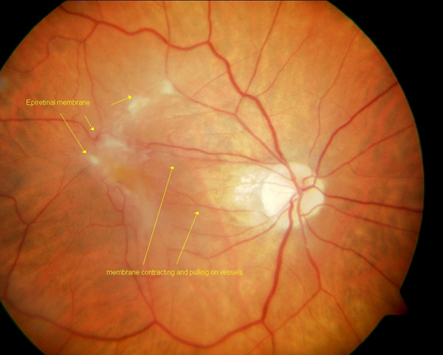
Management of ERM
Early cases - e.g cellophaning but VA isn’t affected and no sx of distortion - manage routinely with a yearly recall
Where VA is affected and/or px reports distortion - refer px routinely
Ask about duration of sx and whether it was sudden/gradual
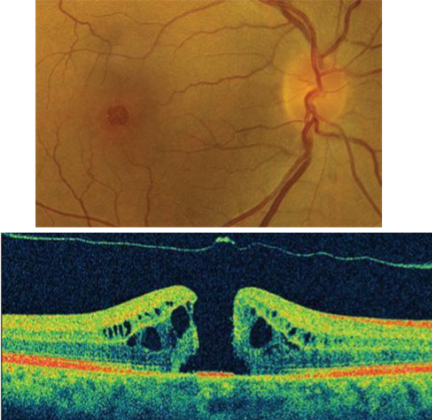
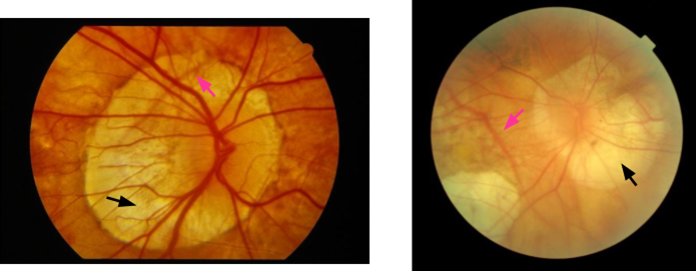
Macular Hole
Adhesions between the vitreous and foveal retinal layers are stronger than the layers themselves - macular hole
Can be thought of as a retinal break that occurs at the macula - limited to the sensory layers of the retina - RPE intact
Association with PVD - therefore very rare in under 60s
More common in males and myopes
Onset of sx weeks/months
Sx of Macular Hole
Vision is compromised - reduced VA and metamorphopsia
Early MH - VA drops by 1-2 lines with slight distorted vision
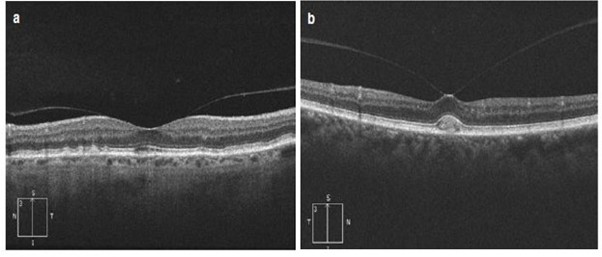
How to confirm if a px has a Macular Hole?
OCT - adhesion attached and raising the macula towards the vitreous
Can perform ‘WAZKE-ALLEN’
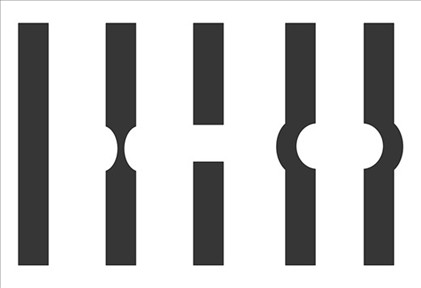
What is Wazke-Allen?
Using a volk lens to make the slit beam 0.5 disc diameter and centered on the macula - ask px to report the perceived shape of the beam - If no macular hole we would expect the beams appearance to match its physical appearance
Signs of early stage MH
Small diameter lesion - small round red/yellow spot/ring at fovea
Decreased/absent foveal depression
Use an OCT to image the break/traction
50% improve themselves if the VRT is resolved
Signs of intermediate stage MH
Moderate diameter lesion
Significant reduction in VA
Distortion likely
Fundus signs more visible
Unlikely to resolve spontaneously
If left untreated - permanent loss of central vision is likely to develop
Signs of end stage MH
Large diameter lesion
Associated with permanent reduction in VA
Positive central scotoma
Less likely to respond to intervention - success of treatment depends on time elapsed since macular hole onset
Optometric management of MH
If confident with diagnosis of either early/intermediate MH refer via GP to be seen within 1/12
End stage MH - routine referral as unlikely to respond to treatment
If unsure about diagnosis then refer suspected MH via fast track AMD pathway
Urgency is a lot lower than RRD as MH can sometimes resolve spontaneously and progression is slower
1 year recall as 15% chance of development of MH in second eye within a 5 year period
Ophthalmological management of MH
Relieving VRT - 90% of recent onset MH with small diameter can be closed by vitrectomy
Intravitreal injection of ocriplasmin - a recombinant protease that helps dissolve the proteins present in the adhesions between vitreous and macular - reduces VRT
Myopic Degeneration
Retina and choroid thinned - susceptible to atrophy - due to increased axial length
At the macula - myopic macular degeneration
Higher the Rx the greater the risk - uncommon in eyes below -6.00D
Sx of MMD
Many cases have no sx
Where they do occur sx similar to dry AMD - gradual, painless onset of blurred central - bilateral (myopia in BE)
In rare cases CNV can develop leading to sudden onset of unilateral blurred/distorted vision - stress on Bruch's membrane = breaks = ingress of CNV = affect sub-RPE and sub-retinal space as with WET AMD
Signs of MMD
PPA
Thinned retina and choroid particularly at the macula
Tilting of the Optic disk
Regions of pallor - washed out fundus - localised areas of hyperpigmentation - subretinal neovascular membrane
Similar to end stage dry AMD - GA
Minority of cases similar to form of WET AMD - CNV
Management of MMD
No effective treatment
Referral only warranted if signs of CNV - urgent referral via AMD pathway
VA has dropped to the point it affects quality of life - routine referral via GP - allows LVA assessment
Hereditary Macular Dystrophies
Stargardt’s disease - most common inherited macular disease
Pattern Dystrophies - ‘Best disease’ - Large egg yoke lesion @macula - lipofuscin build up between RPE and retina - Vitelliform dystrophy
Cone Dystrophy
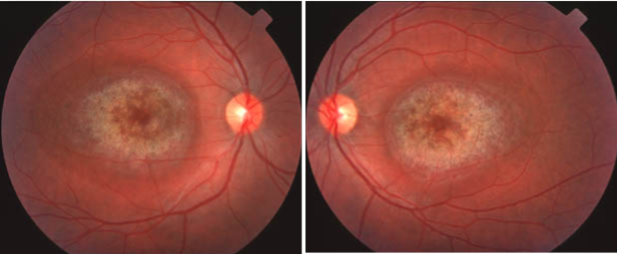
Cone Dystrophy
Can be present at birth (stationary) or progressive
If progressive presents in older childhood/adulthood
Sx include reduced VA and CV
ERG can be used
Late stage fundus - Bull’s eye maculopathy - similar appearance to dry AMD - differentiated by age of px (younger) and the symmetry in BE as it is a genetic condition
Management of Hereditary Macular Dystrophies
If we are unable to ascertain the cause of reduced vision in a child - always refer
Routine referral via GP
Ophthalmology will try confirm diagnosis with ERG and will give LVA assessments