Test 2 - Ch 4 (VSEPR and Molecular Polarity)
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Electron group arrangement
3D geometric shape the bonding and non-bonding e- occupy
Molecular shape
The 3D shape that only the bonded atoms occupy, i.e. what the molecule “looks like” (based on bond angles effect on bonds)
VSEPR notation
AX_mE_n
A - central atom, X - bonded atom, E - L pair
m and n - how many of each you have
X and E are e- groups
Steric #
m + n: total # of “e- groups”
Note: bond order between A and X does not matter. X counts as 1e- group
AX_2
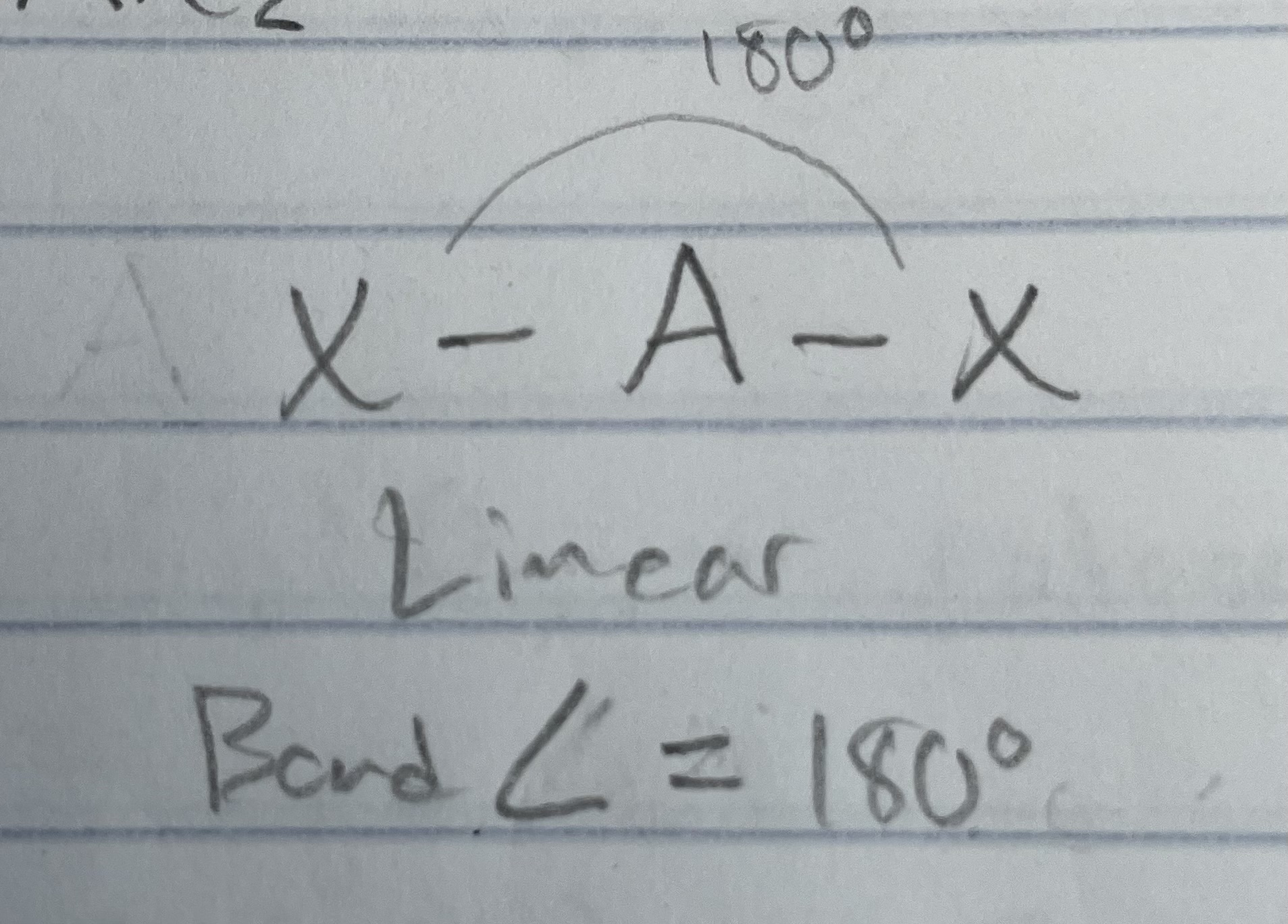
AXE
Note: bond angle N/A bc only 2 atoms (all diatomic are linear)
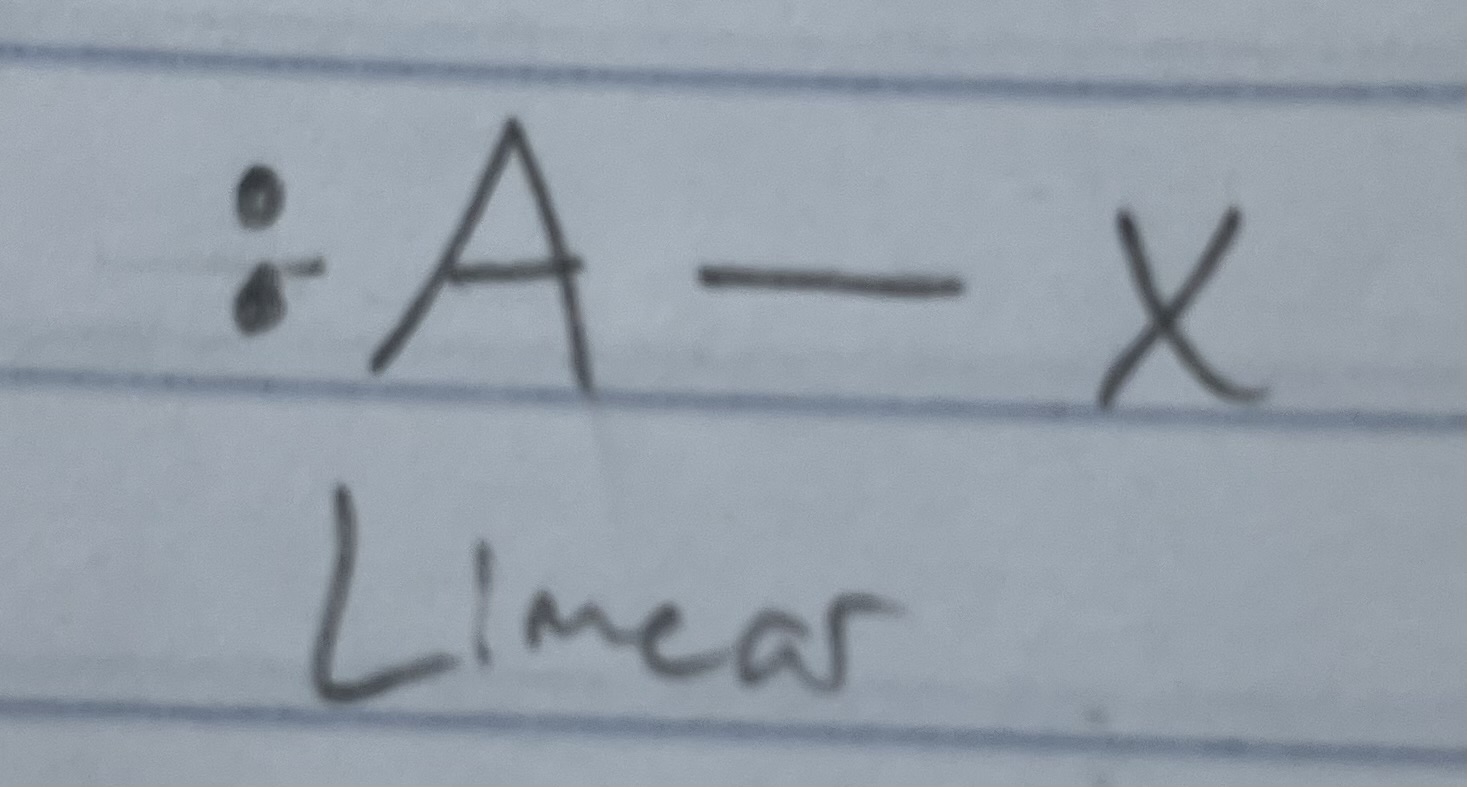
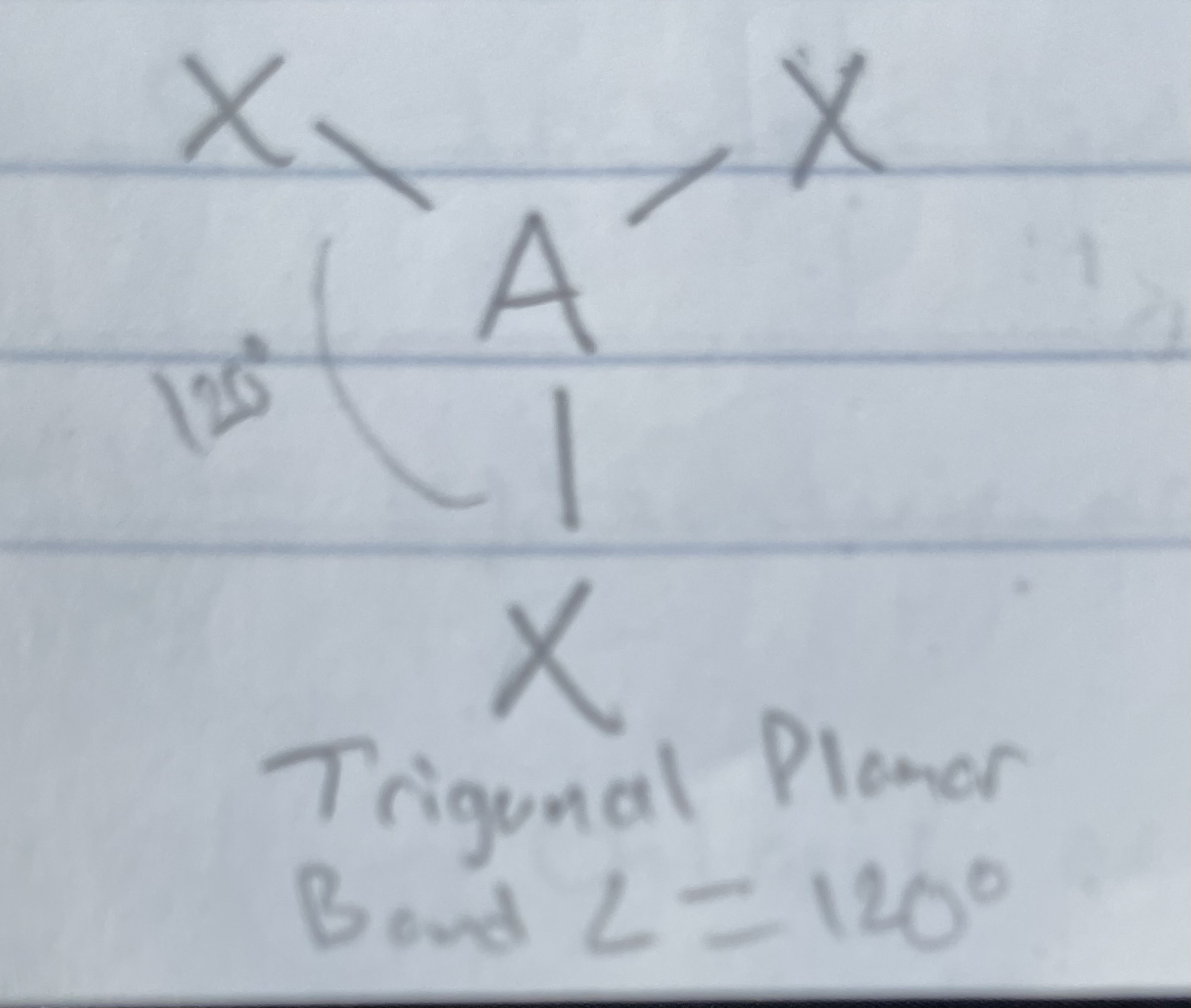
AX_3
Trigonal planar
angle: 120
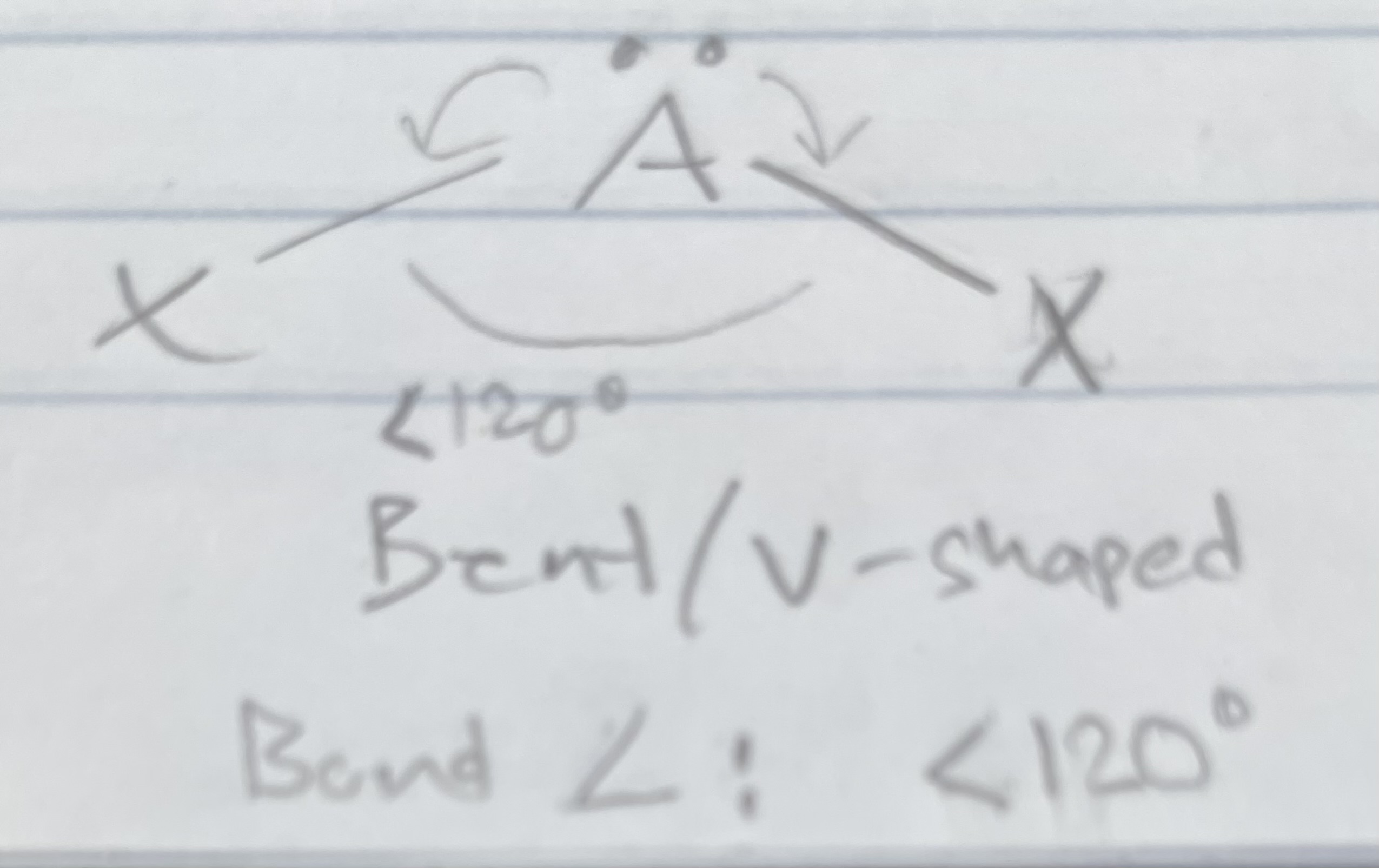
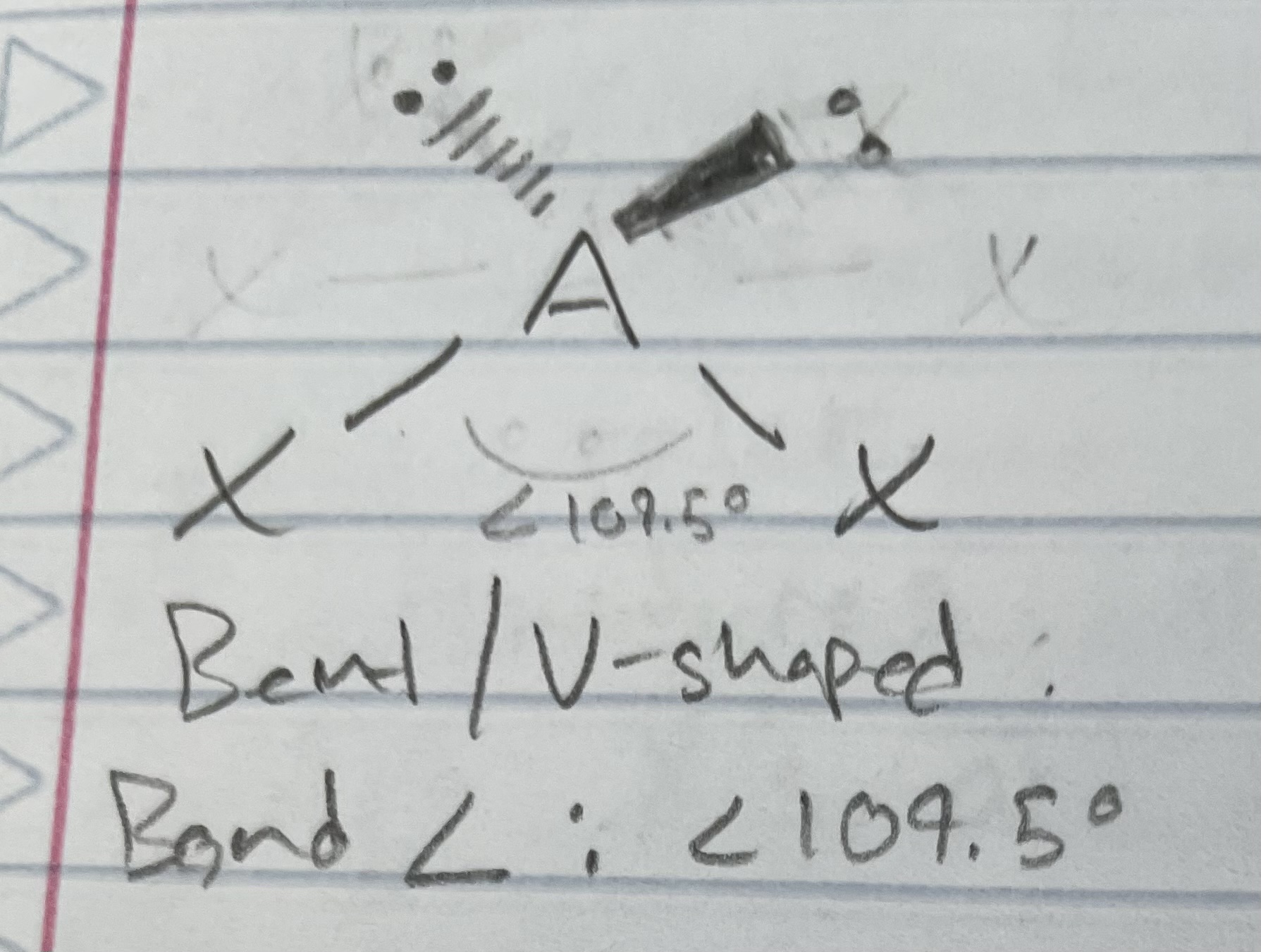
AX_2E
Bent/V-shaped
Angle: <120
AX_4
Tetrahedral
Angle: 109.5
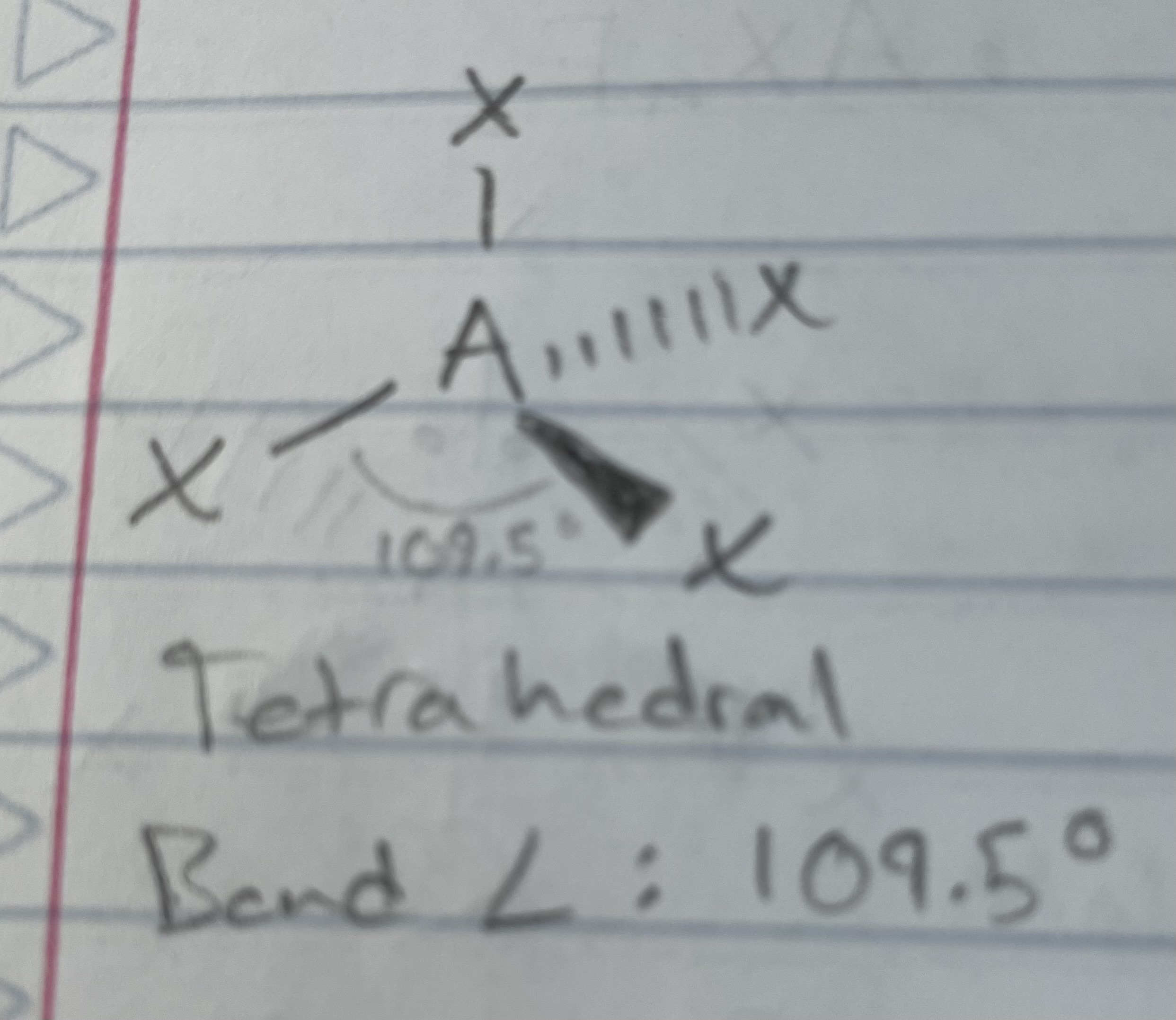
AX_3E
Trigonal pyramidal
Angle: <109.5

AX_2E_2
Bent/V-shaped
Angle: <109.5
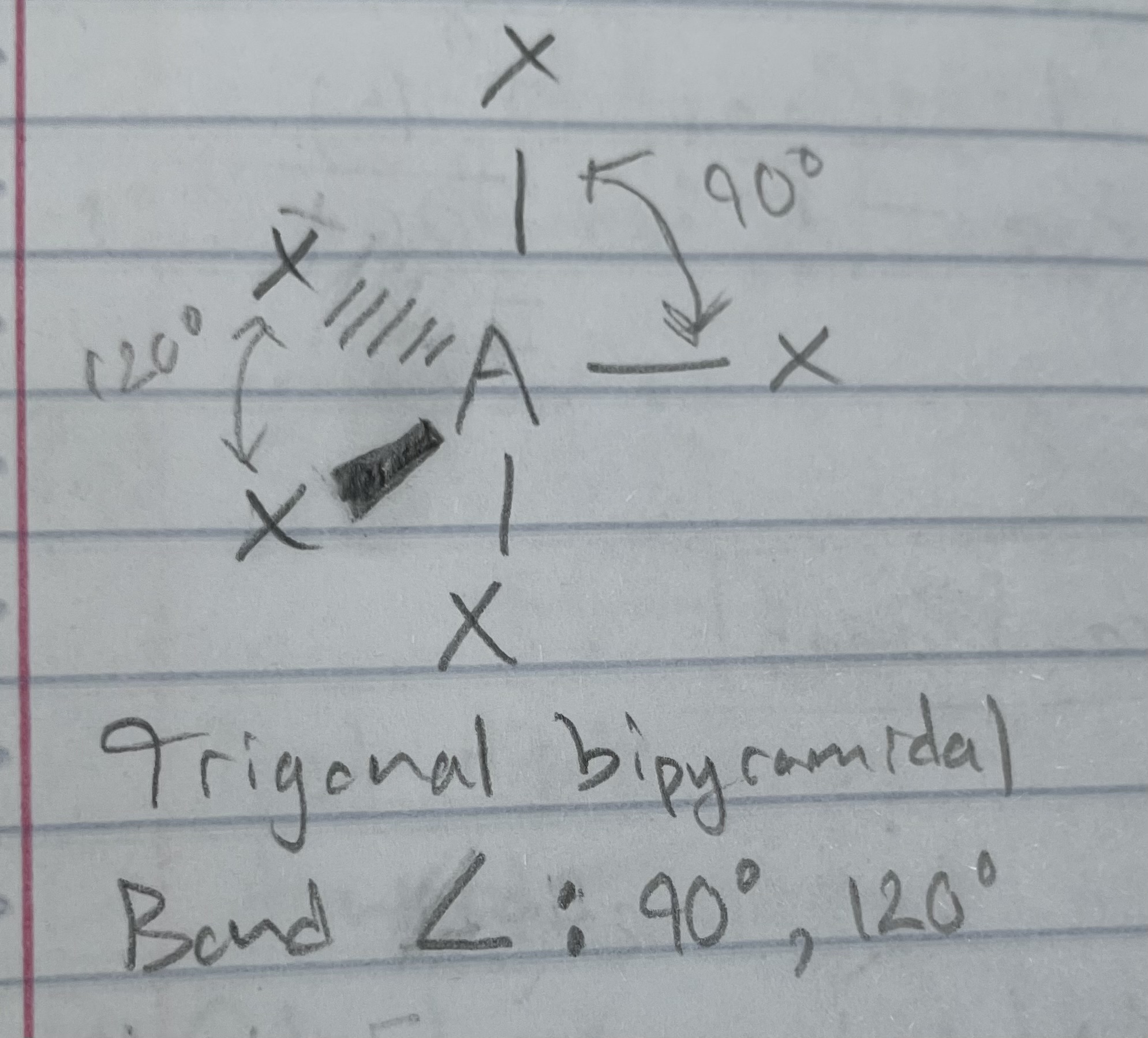
AX_5
Trigonal bipyramidal
Angle: 90, 120
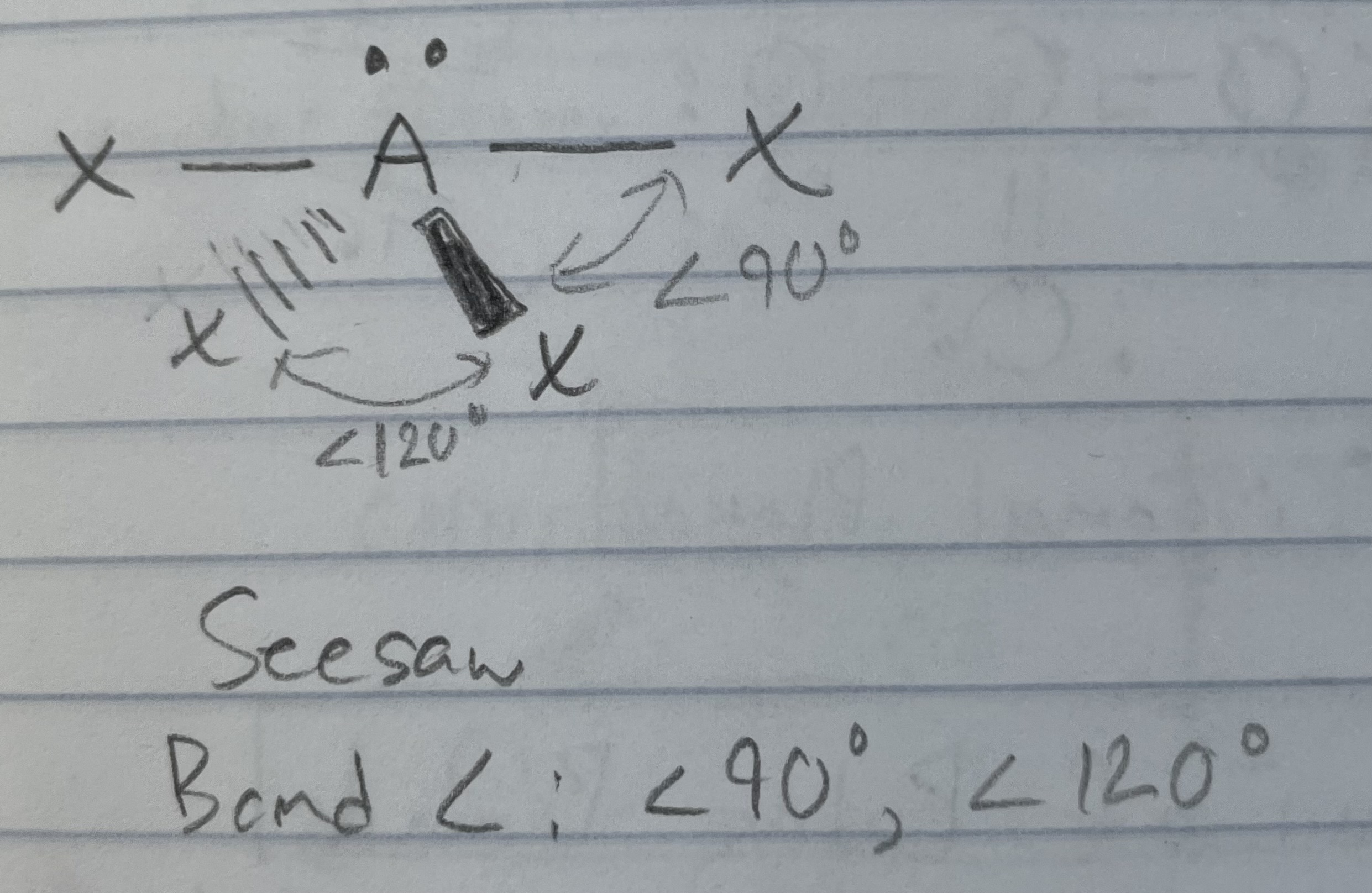
AX_4E
Seesaw
<90, <120
AX_3E_2
T-shaped
Angle: <90
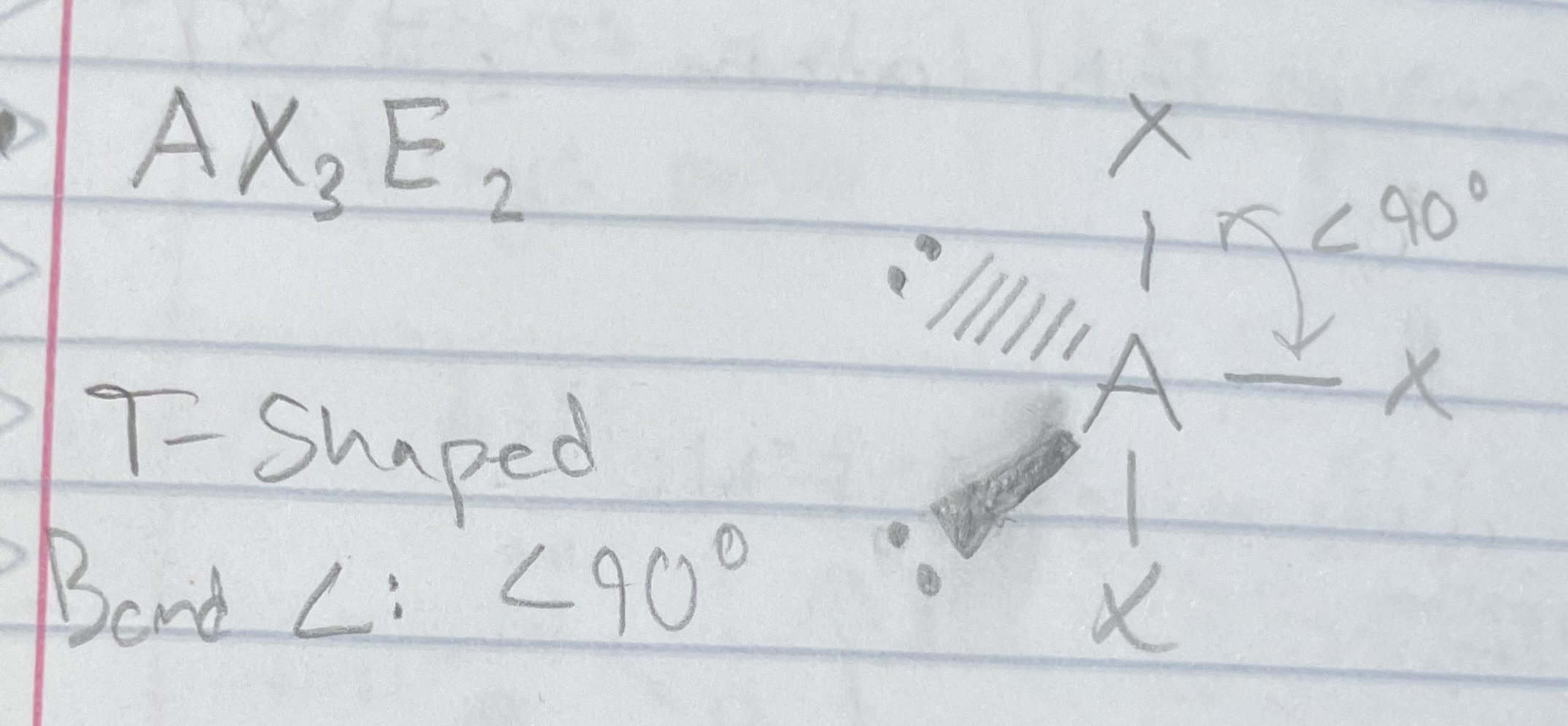
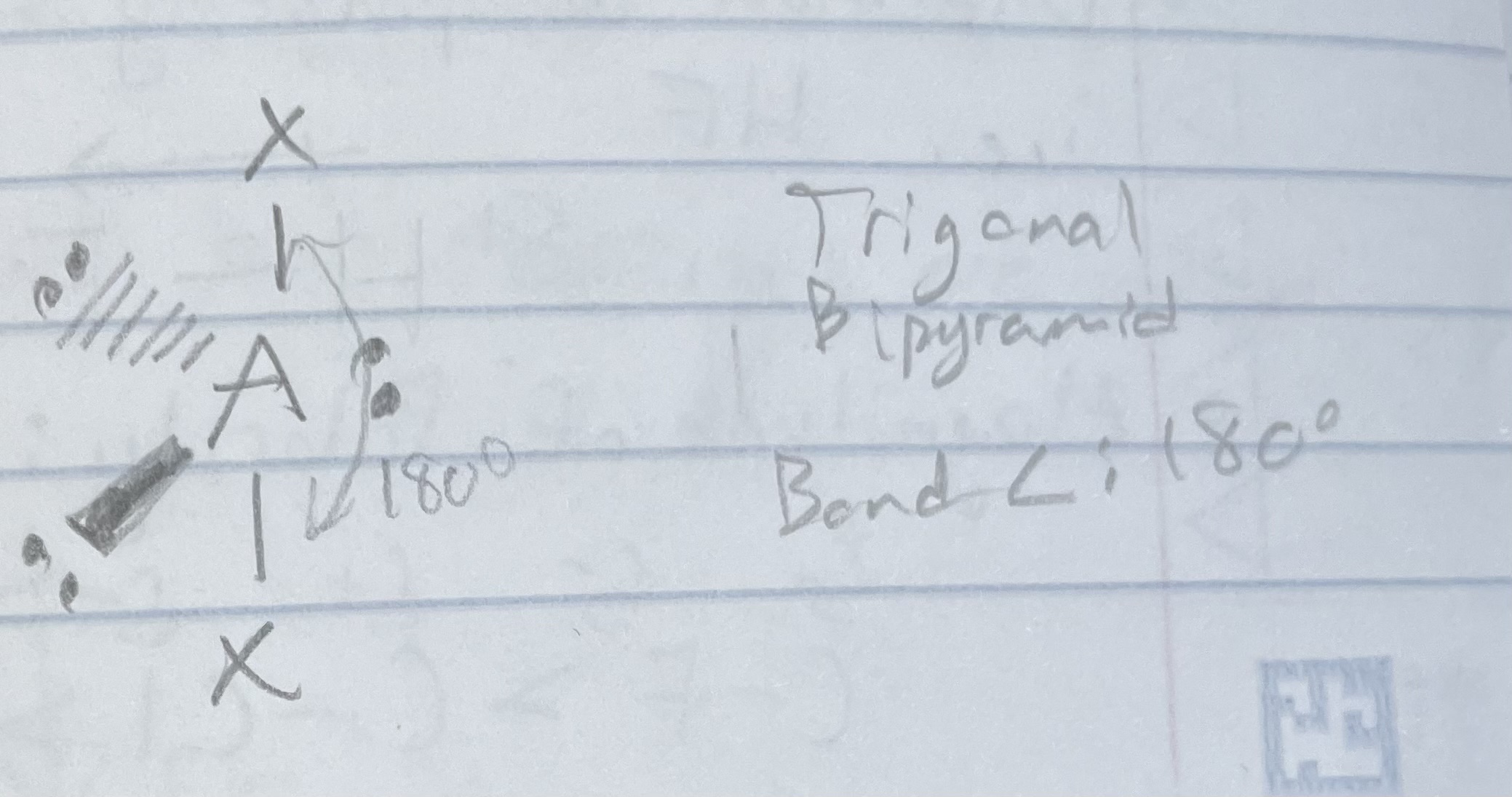
AX_2E_3
Trigonal bipyramidal
Angle: 180
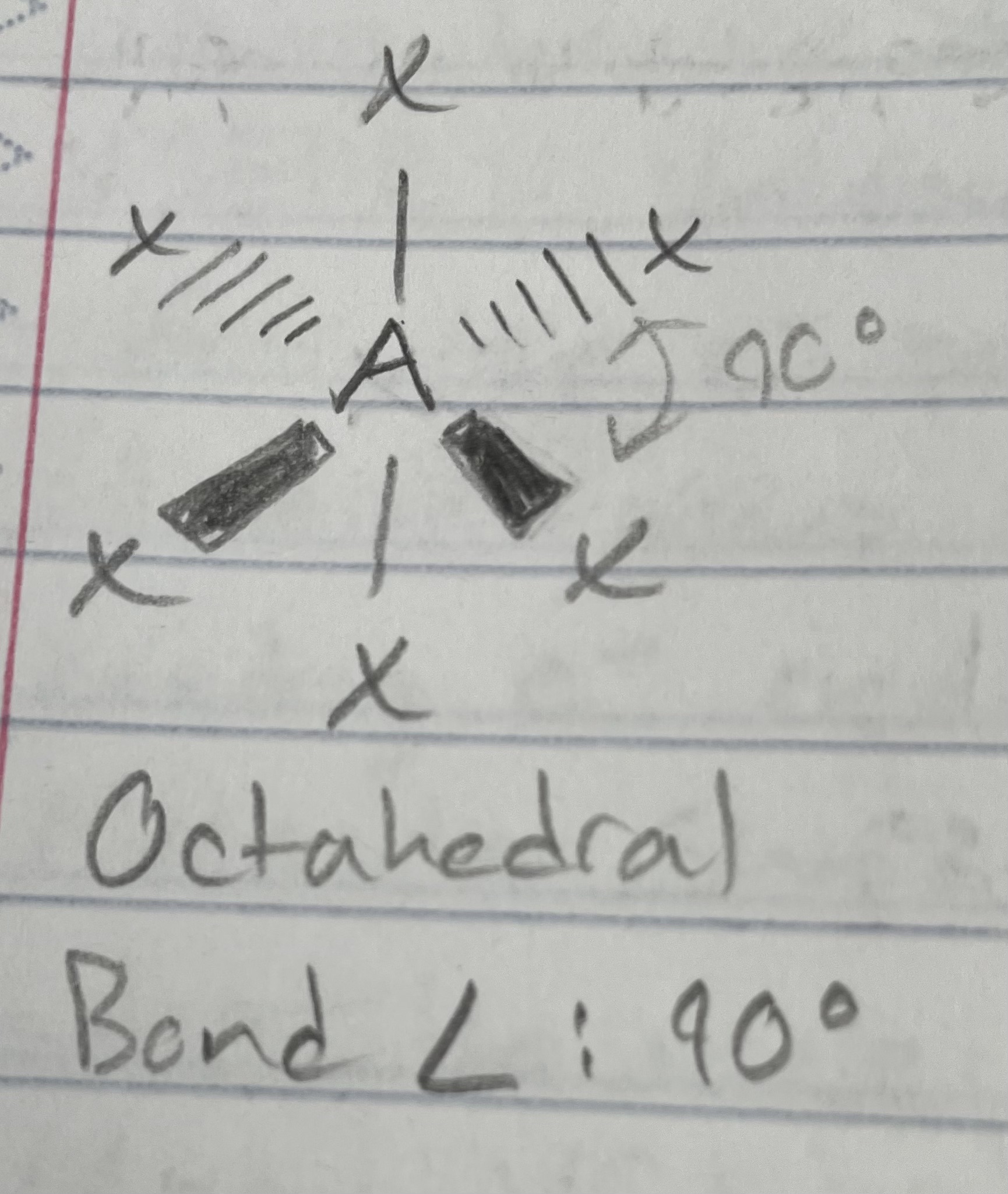
AX_6
Octahedral
Angle: 90
AX_5E
Square pyramidal
Angle: <90
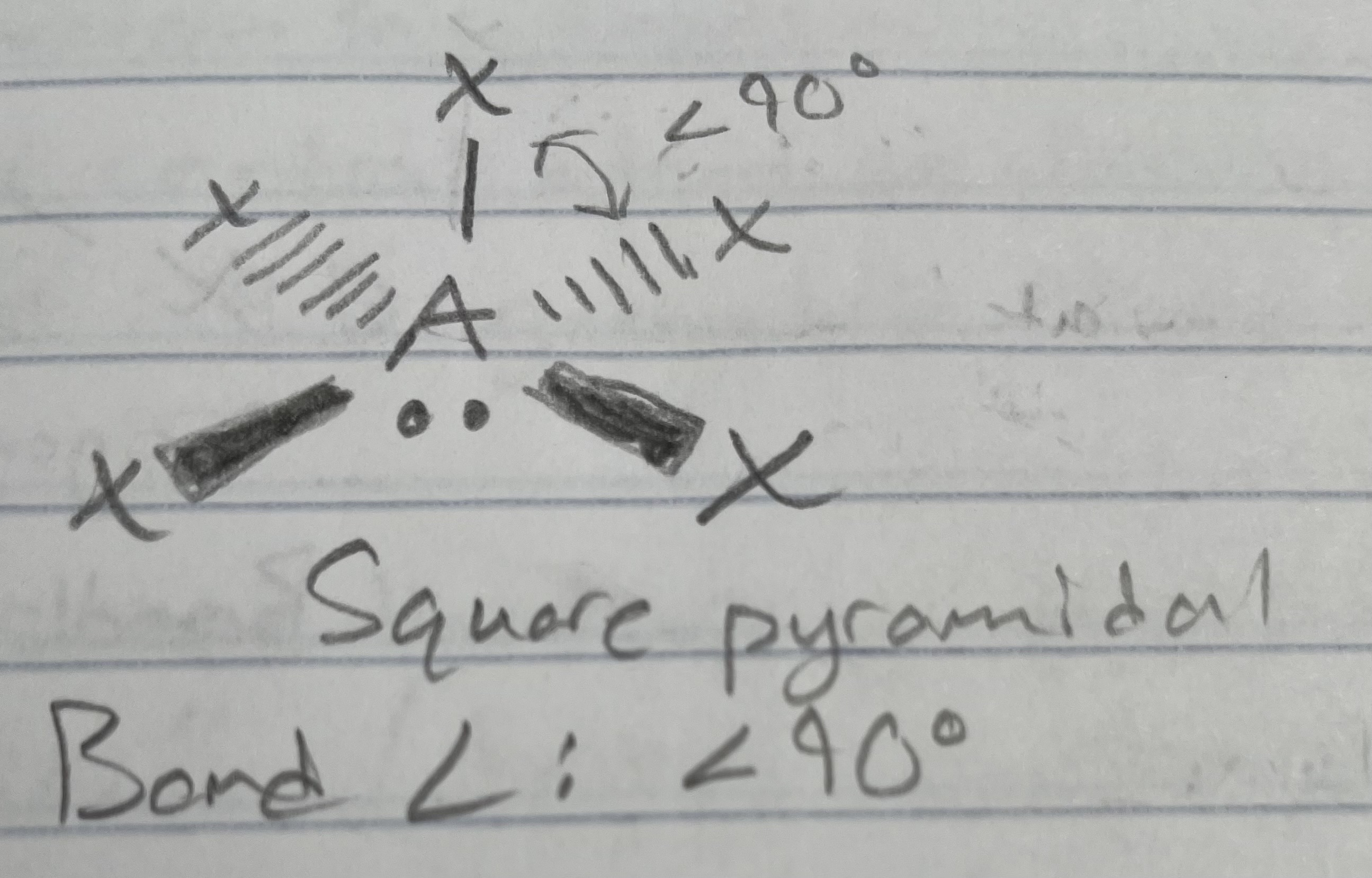
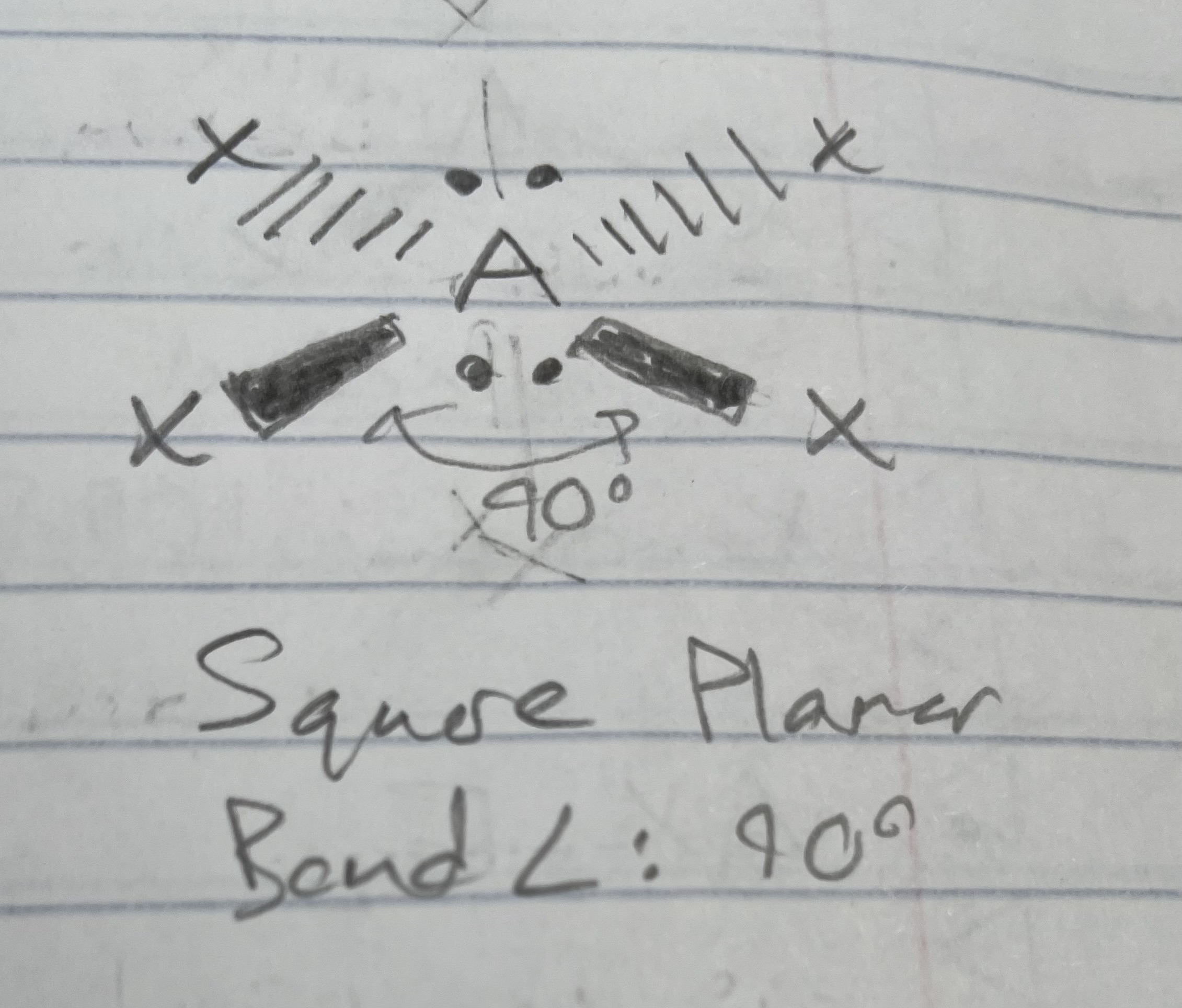
AX_4E_2
Square planar
Angle: 90
Groups that cause bond angle
Lone pairs
Double/triple bonds
Lone pair - lone pair repulsion is STRONGEST
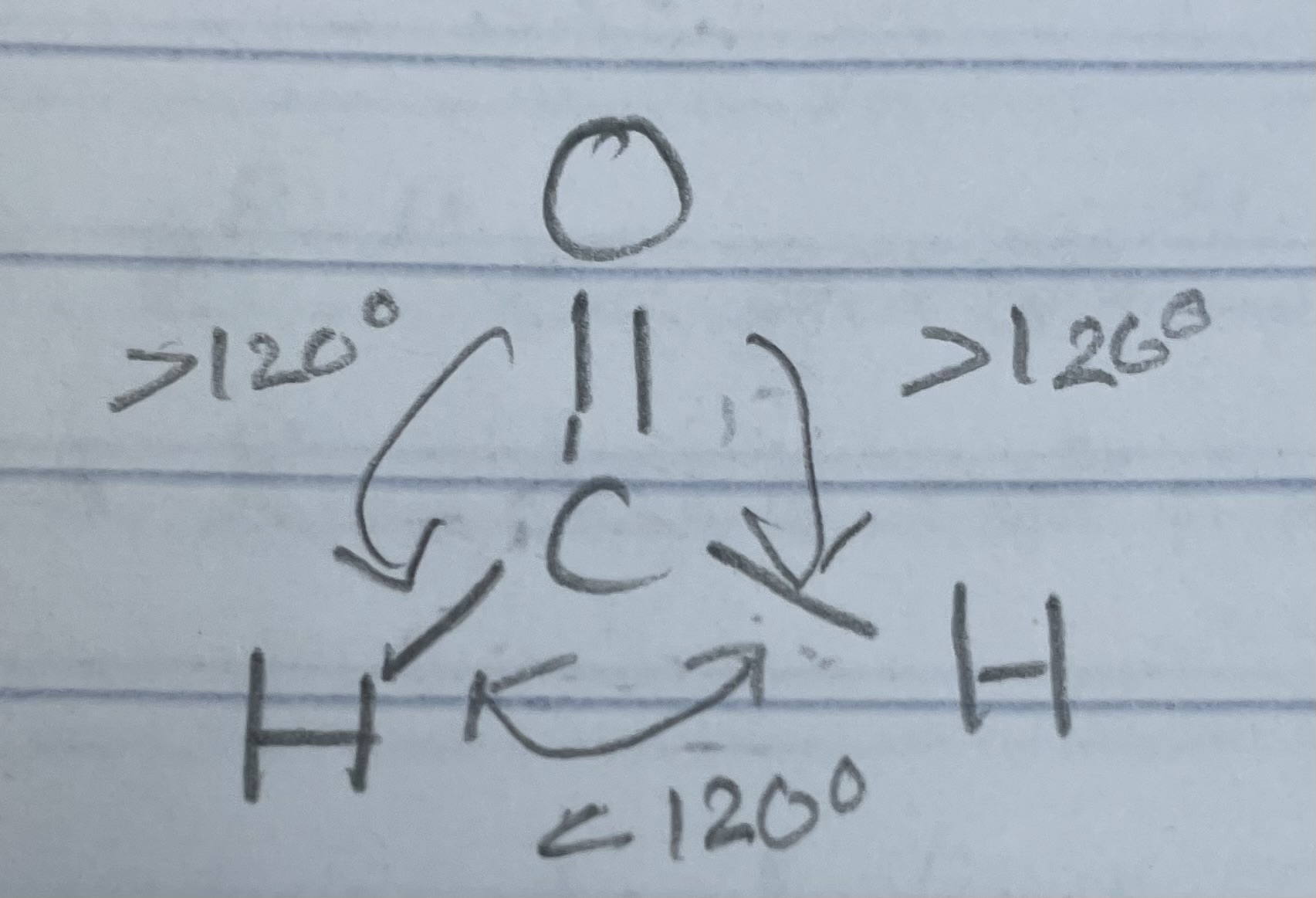
Molecular polarity
An uneven distribution of charge over a whole molecule or large portion
Units: Debye (D) = (3.34E-30) x c x m
Dipole moment abbreviated as M(mu)
Must have polar bonds (Predicting molecular polarity)
Homoatomic molecules (N2, P4, S8, etc.) cannot be polar, but O3 (ozone) is an exception
Geometry is key (Predicting molecular polarity)
The presence of polar bonds does not guarantee that a molecule will be polar, geometry is key
Structures w/ no lone pairs on central atoms (AX_m) (Predicting molecular polarity)
All X’s are the same type of outer atom. All nonpolar
Structures w/ more than 1 type of outer atom (AXX’E_n). Almost always polar
Structures w/ 1 lone pair on central atom (AX_mE_1) (Predicting molecular polarity)
Always polar
-All bent molecules are polar
Structures w/ more than 1 lone pair (Predicting molecular polarity)
May or may not be polar