21 - Viruses in Biomedicine and Biotechnology
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Viruses in science
small genomes
‘simple’ systems
manipulation of cells
pathogens
humans
animals
insects
plants
bacteria
infections
genetics
immunology
cancer
biotechnology
medicine
molecular biology
Application of viruses in medicine and science
genetic engineering → cloning and expression vectors
biological weapons
vaccines
cancer therapy
nanotechnology → viruses as carrier
antibiotics → bacteriophages
agriculture → transgenic animals and plants
gene therapy
viral elements in cloning and expression vectors
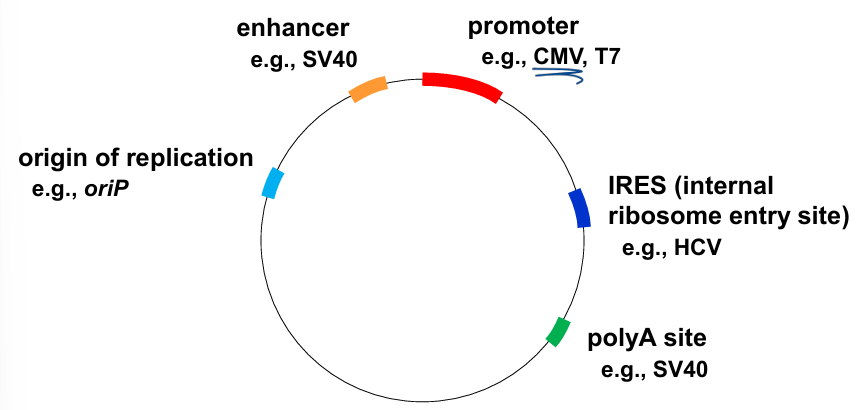
promoter → CMV promoter (human cytomegalovirus major): major TF bind = works in a variety of cells
Enhancer: boosting protein transcription
origin of replication
Internal ribosomal entry site: secondary structure of RNA, interact with 40s subunit
nanotechnology - viruses as carrier
Virus like particles (VPLs)
only capsid/envelope
without genetic material
→ Viruses tolerate a wide range of chemical modifications:
antimicrobial agent
detection
imaging
photosensitive material
drug delivery
biosensor
batteries
tissue regeneration
→ viruses chemically augmented with capabilities limited only by imagination.
example: tobacco mosaic virus TMV
structural feature: helical symmetry → form long and very stable tubes
metal deposition of TMV or its VLPs → metallized TMV or its VLPs used for
nanoelectronics
batteries
catalysts
(bio)sensors
bacteriophages
huge variety, many shapes
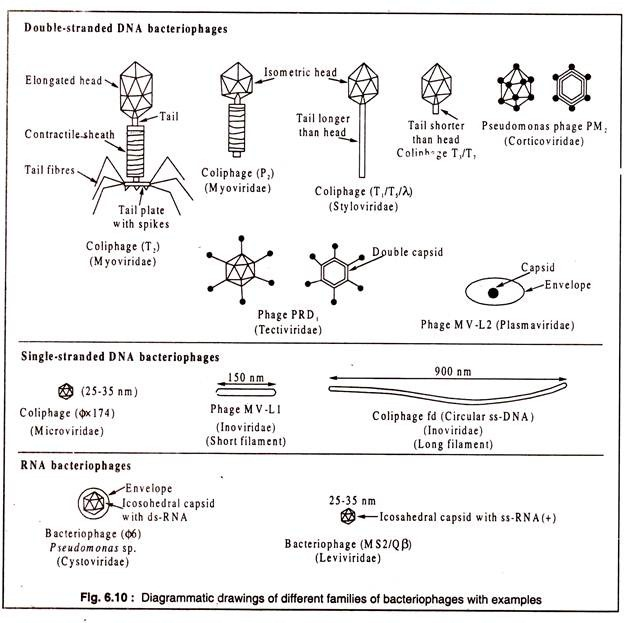
ds DNA, SS DNA, RNA
life cycle: lytic or lysogenic
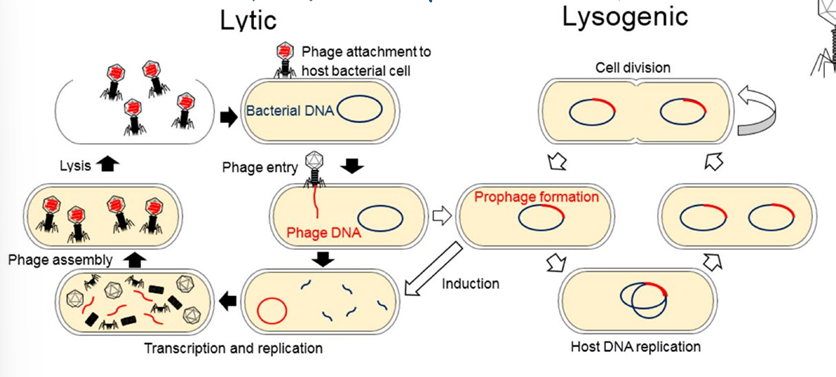
high host specificity → good for antibiotics = less side effects
Factors affecting the effectiveness of phage use against pathogenic bacteria
8
phage administration
phage treatment by: oral, topical, intraperitoneal, intravenous and intranasal administration depending on site of infection
some cases: iv treatment was faster than the intramammary one
phage concentration (MOI)
MOI (multiplicity of infection) = ratio of phage/bacteria
for in vivo and in vitro experiments, MOI varied from 0.01 to 100
dose and moment of treatment
application of phage was most useful when the treatment was early
if treated early, multiple doses are better than a single one
environment conditions
phages survival and persistence affected by physicochemical factors (pH, T)
eg: proliferation of several phages is limited when pH < 4.5
neutralization
by AB or other compounds
need to
repeat the administration
increase the dose
administration of different phages more resistant
protection of phages by encapsulation
accessibility to target bacteria
pathogens develop in tissue or organ compartment inaccessible to phages
phages diffusion limited in solid matrices
immune factors in raw milk could protect bacteria from phages
resistance to phage
bacteria may become resistant → use cocktail of phage or isolate the new phages
specificity
phages must be lytic and able to infect the target bacteria
spectrum of phage activity may be increased by use of a cocktail of phage
gene therapy
basic principles:
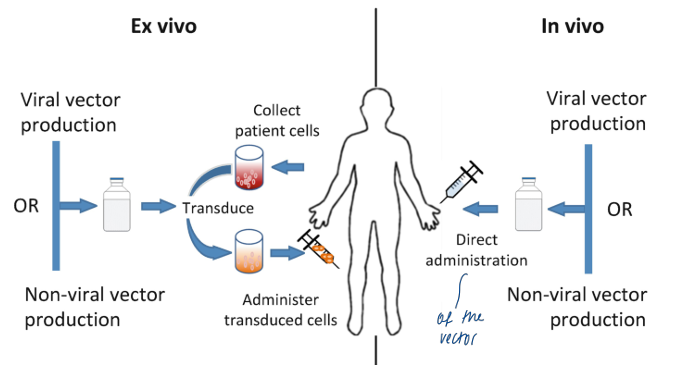
gene augmentation therapy
insert functioning gene in a cell with non-functioning gene
use: monogenic diseases (sickle cell anemia, certain muscle dystrophies, cystic fibrosis,…)
gene inhibition therapy
insert blocking gene in a cell containing faulty gene
use: certain cancers (oncogenes)
killing of specific cells
insert suicide gene in a diseased cell → produce toxic product → cell death
insert marker gene → marker prot on cell surface recognized by IS → cell death
use: cancer/infectionss
Challenges in gene therapy
delivery (right cell, right place, sufficient number of cells targeted, …)
required expression level
duration of desired effect
avoiding immune responses
safety (not the right cell, not the right place, …)
manufacturing and costs
ethical issues
monogenic disease vector treatment vs treat viral infection
monogenic disease vector treatment advantages:
no need to target 100% of cells
replacing only a few is efficient
not the case with viral infections
treat viral infection advantages
no need for lifelong expression
Examples of viral vectors in gene therapy
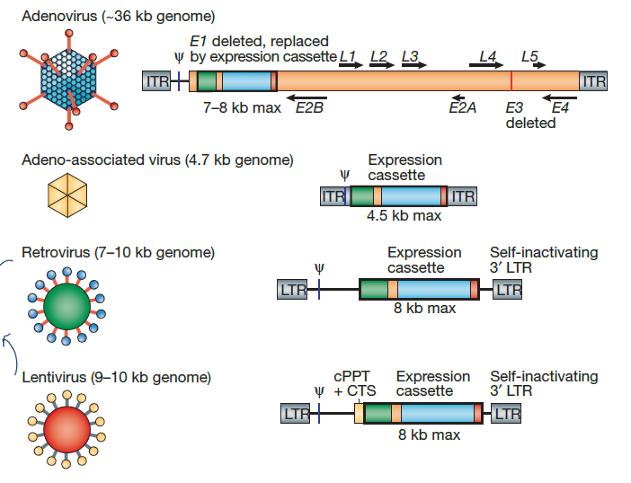
most used:
adenovirus 20.5%
retrovirus 17.9%
naked/plasmid DNA 16.6%
adeno-associated virus 7.6%
lentivirus 7.3%
cancer therapy - Oncolytic viruses
mode of action
modes of action
OV colonizing the tumor
direct OV-mediated tumor lysis
recruitment of immune cells to the inflamed tumor
engulfment of OV-infected tumor cells by DC
AG cross-presentation to specific CTLs
migration of effector CTLs to the tumor site
tumor cell lysis by TAA-specific CTLs
cancer therapy - Oncolytic viruses
OV in the TME
OV in the TME
cancer cell lysis
CAF attack
vascular endothelial cell attack
overcome immune suppression
inflammatory cytokines
antitumor-adaptive immunity
PRR activation
activation of innate IR
immunogenic cell death
combination with other anticancer therapies
cancer therapy - Oncolytic viruses
Barriers to effective OV therapy
→ factors affecting systemic OV delivery
anti-viral serum factors
sequestration by the mononuclear phagocyte system
non-specific binding to red blood cells
high interstitial fluid pressure → inadequate extravasation
→ factors affecting intratumoral OV spread
dense network of ECM
resistance to infection/direct cytotoxicity
infiltrating immune cells
innate anti-viral response (IFN-α/β)
→ factors affecting the generation of anti-tumor immunity
regulatory immune cell infiltration
immune checkpoints (PD-1)
tumor cell downregulation of MHC/expression of immune-inhibitory proteins
aberrant chemokine/cytokine milieu