Atomic Trends in Periodic Tables
1/46
Earn XP
Description and Tags
The whole of Unit 5 - Atomic Number, Atomic Radii, Ionization energy, and Cations and Anions.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
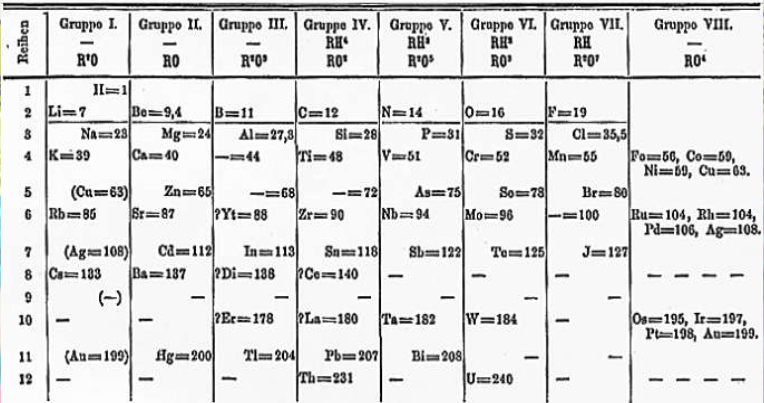
Who created the Periodic Table
Dmitri Mendeleev
How did Dmitri Mendeleev organize the periodic table
By increasing atomic mass
So that elements in the same row have similar properties
Who refined the periodic table
Henry Moseley
How did Henry Moseley rearrange the periodic table?
By increasing atomic number
What we use today
Why is it better to organize the periodic table by increasing atomic number?
Atomic number (protons) determine the element
Helps to identify trends and patterns in properties (periodic law)
Reflects the arrangement of electrons in atoms.
Allows for easy comparison of elements in the same group.
Enables prediction of an element's properties based on its position.
Periodic Law
When elements are arranged in order of increasing atomic number, there is a pattern in their physical and chemical properties
Period
The horizontal rows of the periodic table
Group
Vertical columns of the periodic table
Elements in the same group…
Have similar properties
Groups are numbered from..
1 to 18
Elements in the same period…
Do not have similar properties.
But they have the same number of occupied energy levels
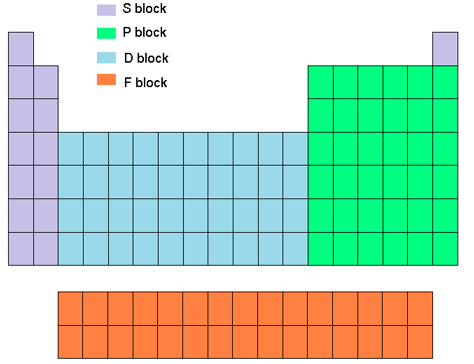
Representative Metals
S & P Blocks
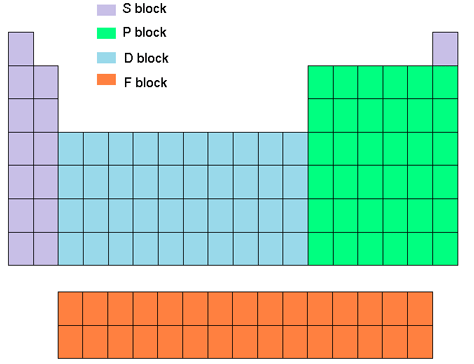
Transition Metals
D Block
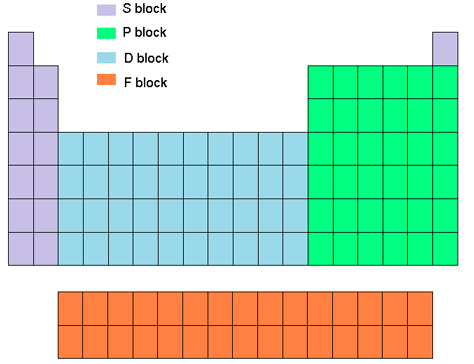
Inner Transition Metals
F Block
What are representative metals?
Alkali metals: Group 1 elements, highly reactive, soft metals
Alkaline earth metals: Group 2 elements, reactive but less than alkali metals
Transition metals:
D-block elements, good conductors of heat and electricity
Metalloids
Share properties between metals and nonmetals
What are inner transition metals?
also known as lanthanides and actinides
are located at the bottom of the periodic table.
occupy f-orbitals in their electron configurations.
These elements possess unique properties and find applications in magnets, catalysts, and nuclear reactors.
what are transition metals
filled in d orbitals.
They are found between alkaline earth metals and nonmetals.
These metals have high melting and boiling points, good conductivity, and can form colored compounds.
They are also known for multiple oxidation states and catalytic activity.
Examples include iron, copper, zinc, silver, and gold.
Trends with atomic size
Atoms do not have fixed radius
The radius of an atom is found by measuring the nuclei in between two touching atoms of the same element and then halving that distance.
Atomic Radius
½ of the radius between two nuclei of two like atoms
Group trend of atomic size
Increases as you go down due to more occupied energy levels
More occupied energy levels = more orbits = greater atomic size
Period trend of atomic size
Decreases from left to right
Shielding effect is constant between periods
Increased protons = increased nuclear charge = electrons are more attracted to center protons = less atomic size
Shielding Effect
The more electrons which are closer to the proton results in outer electrons being repelled due to increase in negative charge.
As negatives and negatives repel —> Outer electrons are repelled and move into farther away energy orbitals.
Thus outer electrons have less attraction to nucleus
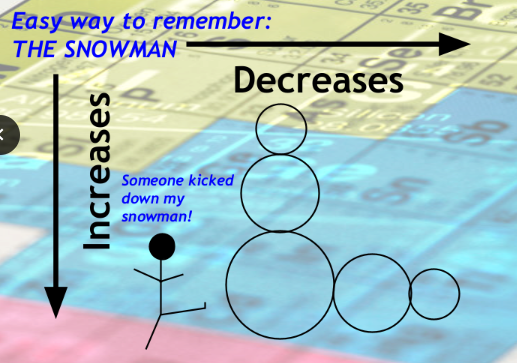
Atomic Size
Increases from top to bottom
Decreases from left to right
How did Dmitri Mendeleev organize the periodic table
Energy required to remove an electron from a gaseous atom
Ionization depends on…
Distance between electrons and nucleus
Nuclear charge (# of protons)
How did Henry Moseley rearrange the periodic table?
By increasing atomic number
What we use today
Period Trend for Ionization Energy
Increases as you go to the right as there is an increased nuclear charge (higher proton number)
More protons = electrons are more attracted to proton
More attraction between electrons and protons = harder to remove electron thus higher ionization energy
Does it require more energy when removing a 2nd or 3rd electron?
Yes, the ionization energy is much higher
As the 2nd or 3rd electron removed from an atom tend to be closer to the proton
Meaning that they will have a higher pull which is harder to break than with the outer and farthest electron
Isoelectronic with a noble gas means that..
There will be a very large increase of ionization energy when an electron is very
Properties of Nonmetals
Generally tend to be the opposite of metals
Brittle (breaks easily)
Dull
Poor conductors of heat or electricity
Good insulator
Why is it that atoms isoelectronic to noble gases have high ionization energy levels?
Isoelectronic with a noble gas means having the same number of electrons as a noble gas.
Noble gases have full electron shells, making them stable.
Removing an electron from an isoelectronic species disrupts this stability, requiring a significant amount of energy
Ionization energy increases substantially.
Cations Ionic Size
Smaller than neutral atom from which they were made from
Why are cations smaller than their neutral atoms?
Loss of energy levels (loss of orbitals = less rings around atom)
More protons than electrons means that more electrons will be pulled closer to nucleus
Cation Ionic Size trends
The more electrons lost, the smaller the ion becomes
Anions Ionic Size
Anions are always larger than their neutral atoms
Why do anions increase in size than their neutral atoms?
More electrons than protons result in less attractive force to proton
Electrons that are less attracted to protons will be farther away
Increase in electrons = more electron orbitals
Properties of metals
Lustrous (shiny)
Good conductors of heat and electricity
Malleable (Example: Aluminium can be split into thin sheets)
Ductile (Can be turned into metals)
Anion Ionic Size Trends
The more electrons gained, the bigger the ion becomes
Electronegativity
Ability for an element to attract other electrons when a compound (when it is chemically combined with another element)
Highest electronegativity level
4.0
Group trends for electronegativity level
Decreases as you go down
Why does electronegativity decrease as you go down?
More electrons = more electrons farther away from nucleus
Less electrons attracted to nucleus = less electronegativity
Period trend for electronegativity
Increases from left to right
Why does electronegativity increase across a period?
More nuclear charge (more protons) = electrons will be more attracted to nucleus