Toxins
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
In livestock, typically, exposure is through what?
contaminated feed/water or too high a dose of an otherwise safe feed/water additive/med
access to poisonous plants, pesticides, and rodenticides
envenomation
exposure to noxious gases
topical application
what are household pets more likely to be exposed to
human-associated hazards
how are toxins typically diagnosed
based on hx of exposure, signs, and lesions on post-mortem
how are toxins generally treated
removal of toxin and supportive care, potentially lavage or “flush” GIT if ingestion was recent
induce vomiting for some recently ingested toxins (not recommended in livestock)
if topical bathe animal
specific commercial tx → general binding agents or anti-toxins
what is the primary concern with toxicosis
dose- exact amount of exposure/intake
what is the amount of toxin required to cause pathology generally correlated to
body weight
what other factors are important for toxicosis
duration and frequency, route of exposure
what are variations in toxicosis
absorption, metabolism, excretion, nutrition/dietary factors, hormonal/health status, organ pathology, stress, environmental factors
samples collected for potential toxicosis analysis include what
dead or recently euthanized animals tha showed signs
feed and drinking water that was available when signs were present
label or picture of product/plant/animal that is suspected to have caused toxicity
what are the two families of venomous snakes in North America
Elapidae- coral snakes
Crotalidae- pit vipers (rattlesnakes, copperhead, cottonmouth moccasin)

what is the only venomous snake native to NH
timber rattlesnake

where do livestock typically get bit by snakes
tongue, nose, face

what are signs for elapid envenomation
systemic neurologic signs → paresis, ataxia, depressed gag reflex w drooling, muscle twitching, quiet mentation, rapid shallow breathing
pain/swelling at the bite are minimal
what are the signs for crotalid envenomation
severe local tissue damage that spreads → tissue discoloration w/i a few minutes, bloody fluid oozing from wounds, skin may slough
neurologic less likely, mostly muscle twitching
what does the prognosis for snake bites depend on
type/species of snake
location of bite
size of victim
degree of envenomation
time between bite and start of tx
what can tx include
preventing/controlling cardiovascular shock
neutralizing venom
preventing or controlling clotting issues
minimizing tissue necrosis
prevent secondary infection
what is the only direct and specific means of neutralizing snake venom
antivenom → if administered in first 6 hr after bite
what mechanisms can rodenticides act through
anticoagulants
neurotoxins
kidney failure
multisystem organ failure
what do anticoagulant rodenticides do
interfere with ability of blood to clot → internal hemorrhage (lungs, intestines, body cavity)
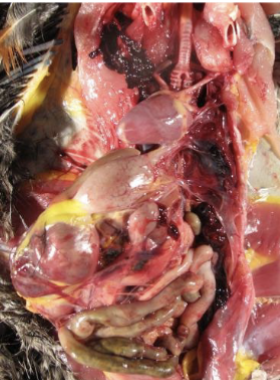
how may anticoagulant rodenticides be treated
with vit K if exact product known
what is the increased susceptibility of skin to damage caused by UV light
photosensitization
during photosensitization, what accumulates in the skin
photodynamic chemicals → stimulated by sunlight on exposed and non pigmented areas
what can photosensitization cause
damage to small vessels by free radicals → skin necrosis and sloughing
what is photosensitization most common in
cattle, sheep, goats, horses
what does the time interval between exposure to photodynamic agent and onset of signs depend on
type of agent
dose
exposure to sunlight
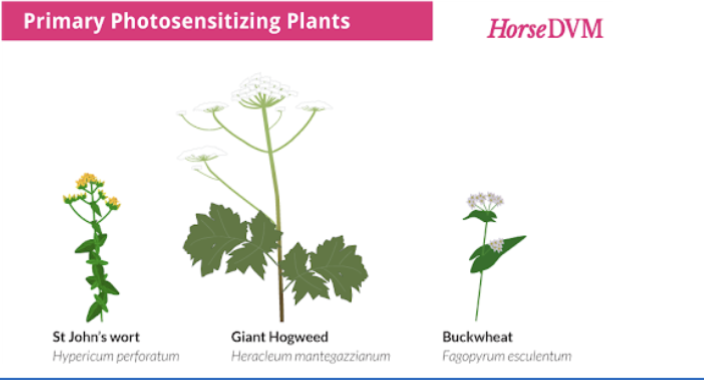
what photosensitization occurs when the photodynamic agent is either ingested, injected, or absorbed through the skin
primary, results in skin cell membrane damage

what is an example of primary photosensitization
hypericin from St. John’s wort and fagopyrin from buckwheat
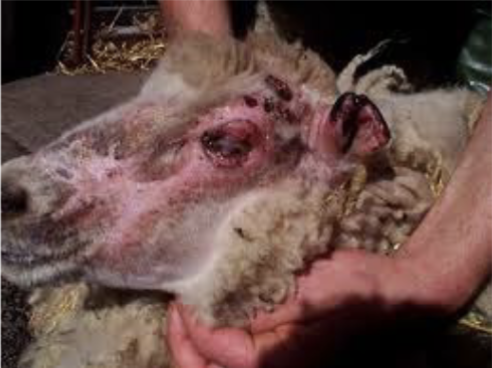
what photosensitization causes liver damage that results in accumulation of photodynamic substances
secondary → more frequent
what are plant derived photosensitizing agents
phytophorphyrins
what are signs for photosensitization
head shaking, restlessness, redness of unpigmented skin, edema/swelling of eyelids, muzzle, ears, and tail
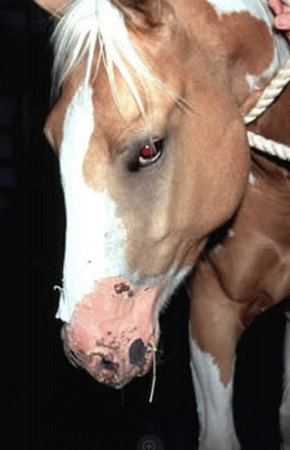
what photosensitization has better prognosis
primary > secondary
tx for photosensitization
remove offending substance
manage complicating infections ± liver damage
provide shade- graze at night
what comes from exposure to feed/bedding contaminated with toxins produced during growth of various fungi/molds on cereals, hay, straw, pastures, etc.
mycotoxins
how are mycotoxins diagnosed
presence of a dz documented to be cause by a known mycotoxin, detection of mycotoxin in feed or animal tissues
some mycotoxins are what (allow pathogens to create secondary disease that complicates diagnosis)
immunosuppressive
what to do if suspected mycotoxicosis
change feed
inspect storage bins, mixing equipment, feeders for caking, molding, or musty odors
clean equipment and sanitize with dilute bleach
analyze for known mycotoxins → spore counts, fungal cultures
use commercially available mold inhibitor, mycotoxin adsorbent if appropriate
save sample of each diet until animals are at 1 mo beyond when the feed was consumed
methods of mycotoxicosis prevention:
testing of suspect feed at harvest
maintaining clean/dry storage facilities
using additive to control mold growth in storage
efficient air exclusion in silage storage
reduce storage time of prepared feeds
what is produced by toxigenic strains of Aspergillus flavus and A parasiticus, is common on peanuts, soybeans, corn, etc when moisture content and temp are sufficiently high
alfatoxicosis
what species does aflatoxicosis affect
growing poultry (especially ducklings and turkey poults), pigs, calves, and dogs
Where is alfatoxicosis metabolized
liver → high dosages cause necrosis of liver cells, prolonged low dosages result in reduced growth rate, immunosuppression, liver enlargement
what does acute aflatoxicosis entail
death after short period of inappetence, vomiting, hemorrhage, jaundice
what does chronic aflatoxicosis entail
more common, unthriftiness, weakness, anorexia, reduced growth and feed efficiency, occasional death
how is aflatoxicosis diagnosed
dz hx, microscopic examination of liver (biopsy or necropsy), presence and levels of aflatoxins in feed should be determined
what is the ingestion of sclerotia of parasitic fungus Claviceps purpura
ergotism
what grains/forage plants are related to ergotism
rye, bluegrasses, fescues, ryegrasses

what species are most commonly involved in ergotism outbreaks
cattle, sheep, pigs, poultry, but most other species are susceptible
what does ergotism primarily cause (sign)
vasoconstriction by direct action on muscle of arterioles, repeated doses injures blood vessels → initially reduce blood flow and eventually leads to necrosis of extremities
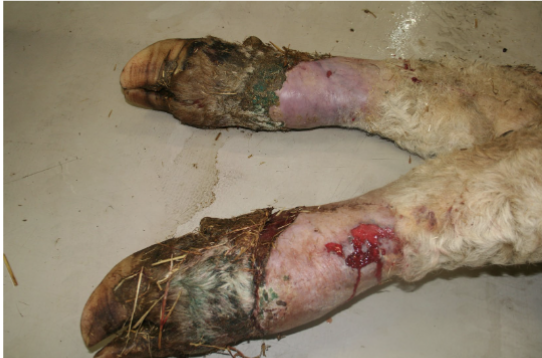
how does ergotism appear in ruminants
lameness (2-6 wk or more after initial ingestion), hindlimbs b4 forelimbs, within ~1 wk, sensation is lost in affected part the dry gangrene sets in
tip of tail or ears become necrotic and slough off
body temp, pulse, respiration rates increase
stimulation of CNS followed by depression (sheep)
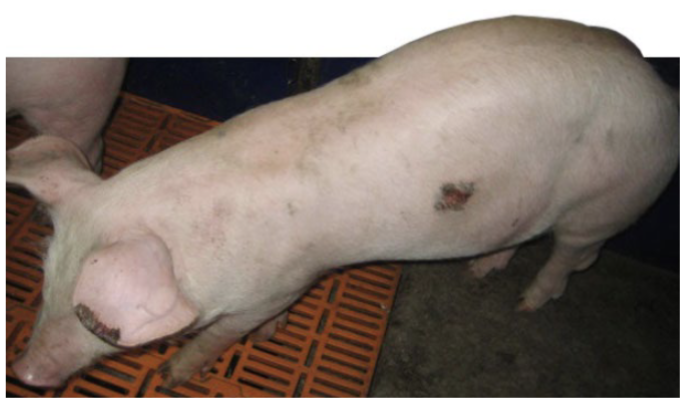
how does ergotism present in pigs
reduced feed intake and weight gain
occasionally necrosis of tips of ears or tail
in pregnant gilts/sows → lack of udder development w agalactia at parturition, piglets smaller and starve to death w/i few days
how is ergotism diagnosed
find causative fungus in grains, hay, pastures provided to livestock showing signs
control for ergotism
immediate change to ergot-free diet, under pasture conditions, frequent grazing or topping of pastures prone to ergot infestation during summer months reduces sclerotia production
what species are particularly sensitive to copper toxicosis
sheep
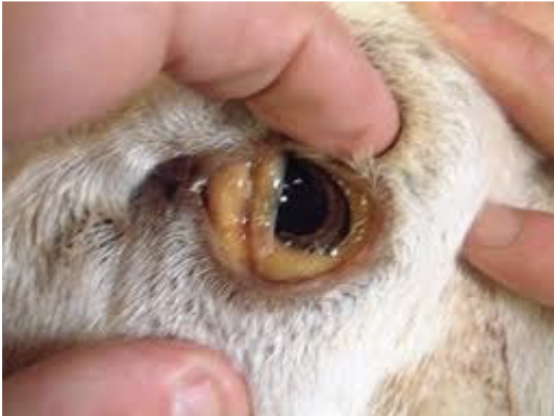
copper toxicosis signs
sudden release of a large amount of copper from liver to blood → stress and liver damage or disease (e.g. flukes) → hemolysis = anemia with jaundice
depression, anorexia, diarrhea, weakness
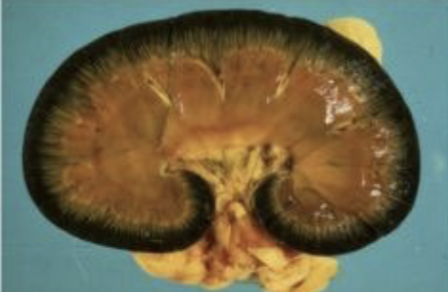
copper toxicosis lesions
most tissues will be yellow, but kidneys are black because of accumulation of RBC breakdown products
how is copper toxicosis diagnosed
measure Cu level in blood or kidneys
copper toxicosis tx
usually unrewarding, fluid therapy, supplemental molybdenum, minimize stress
Copper toxicosis prevention
feeding 5-10 ppm of Cu in total diet and less than a 10:1 ratio of copper to molybdenum
what is polioencephalomalacia
edema, swelling and necrosis of brain gray matter, may cause brain to herniate
signs of polioencephalomalacia
ataxia → recumbency, head pressing, central blindness, opisthotonos, muscle tremors, seizure, nystagmus, decreased mentation
causes of polioencephalomalacia
thiamine deficiency, sulfur toxicosis, lead toxicosis, salt toxicosis
what deficiency is most often associated with rumen acidosis
vit B1 (thiamine)
how is vit B1 deficiency prevented
provide good quality roughage, slowly adapting to diet changes, avoiding toxic plants
possible feed sources for sulfur toxicosis
gypsum, urinary acidifiers, molasses, cruciferous crops, water
what toxicosis makes the rumen smell like eggs as a result of H2S gas production
sulfur
diagnosis for sulfur toxicosis
demonstrate exposure and possibly by measuring H2S
sulfur toxicosis tx
no specific tx, remove offending substance and supportive care
what are environmental lead contaminants
insecticides, herbicides, lead-acid batteries, lead paint, gasoline, shot gun pellets
lead toxicosis mechanism
once absorbed, lead irreversibly binds to RBCs, deposits in bones, kidneys, and liver, can cross placental border
lead toxicosis signs
CNS, GI irritation (colic, anorexia, diarrhea), osteoporosis (lameness, paralysis)
how is lead toxicosis diagnosed
measure blood or tissue lead levels
lead toxicosis tx
removal of ingested toxin and chelation therapy (binds lead in blood and aids in excretion)
term for excessive salt concentration in blood
hypernatremia
what species is most sensitive to salt toxicosis
swine, mortality >50%
how to control salt toxicosis
slowly bring down salt concentration in blood, controlled access to fluids over multiple days