Epilepsy and Sleep Disorders PY367
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
What is sleep?
Closed eye with reduction in body movement and electromyographic activity, responsiveness to external stimuli, breathing rates, altered body position, and brain wave architecture (polysomnography)
What is REM sleep?
Starts after 90 minutes after sleeping and increases in about 10 minutes every 90 minutes. So sleeping for longer periods of time increases the duration of REM cycles. REM cycles are associated with dreaming, cholinergic and glutamatergic neurons are maximally active, while monoaminergic neurons are less active.
What is the sleep pathway?
· The hypothalamus contains sleep-promoting neuronal populations: the ventrolateral preoptic area (VLPO) and median preoptic area (MNPO)
· VLPO and MNPO primarily express the inhibitory neurotransmitters GABA and galanin, and project to the wake-promoting system
· Adenosine has been shown to accumulate in VLPO
· There are four adenosine receptors: A1R, A2AR, A2BR, and A3R
· Adenosine and activate adenosine receptors (A2AR) of VLPO and hence activating inhibitor neurons that inhibit ascending pathway
· Moreover, adenosine activate the inhibitory receptor A1 expressed on ascending arousal areas such as BF
· Caffeine is adenosine receptor antagonist
VLPO receives signalling inputs from suprachiasmatic nucleus
Why are many neurotransmitters involved in sleep?
If one neurotransmitter is involved, if inhibited, problems may arise so many neurotransmitters are involved to maintain sleep
What are the ARAS neurotransmitters?
Ascending reticular activating system. These neurotransmitters need to be suppressed to induce and maintain sleep.
· Dopamine from the ventral periaqueductal gray matter
· Excitatory noradrenaline (Norepinephrine) arising from the locus coeruleus
· Serotonin from the midline raphe nuclei
· Acetylcholine from the pons
· Histamine from the tuberomammillary nucleus
Orexin from the perifornical area
What are the basic treatments for sleep disorders?
· NARCOLEPSY (excessive sleeping): enhance ARAS
INSOMNIA (less sleeping): suppress ARAS
How is dopamine synthesised?
· Dopamine (DA) is synthesised from amino acids:
- Tyrosine (mainly obtained from diet)
- Phenylalanine (from the liver)
· Tyrosine is the precursor amino acid in catecholamines synthesis
· DA is synthesised from L-tyrosine in the cytoplasm of the neurons
· DA is then transported into secretory vesicles for storage and release
The insertion of DA into vesicles is achieved through pumps:
- Proton ATPase pumps that facilitate H+ influx into the vesicle
- Proton antiporter: vesicular monoamine transporter (VMAT) that works as dopamine/H+ exchanger
What is the activity of Dopamine?
· After the synthesis, DA is stored in the vesicle of DA neurons in the ventral periaqueductal gray matter (vPAG) near the dorsal raphe nucleus
· Action potential induces calcium influx and dopamine release
· DA binds to DA-receptors (D1 mainly) in the postsynaptic affected
DA binds to DA-autoreceptor (D2 types) in the presynaptic affected organ/tissue leading to inhibitory effect (negative feedback)
How is dopamine activity terminated?
From the cleft DA is
· Transported from the cleft back into the presynaptic neuron by selective dopamine-sodium co-transporter (DAT) to either recycled in the vesicle, or metabolised by mitochondrial monoamine oxidase (MAOA or MAOB)
· Metabolised by cleft catecholamine-O-methyltransferase (COMT)
Homovanillic acid (HVA) is the major metabolite of DA, which is excreted in the urine
What are the 4 main pathways dopamine is involved in?
· Mesolimbic pathway: an excess of DA in this pathway has been linked to psychosis and the positive symptoms of schizophrenia such as hallucinations and delusions
· Mesocortical pathway: problems with DA function in this pathway may be responsible for the negative symptoms and cognitive deficits such as flattening of emotion and reduction in attention and working memory
· Nigrostriatal pathway; involved in motor control and Parkinson’s Disease
Tubero-infundibular pathway; involved in hormone secretion.
What are the effects of MAOi on sleep?
Phenelzine (insomnia) and moclobemide (sleep disorder) (MAOi): inhibits metabolism of dopamine so more dopamine available in system so causes insomnia
What are the effects of dopamine agonists on sleep?
Bromocriptine is dopamine agonist and prolactin inhibitor which causes sleep disorder: induces dopamine receptors and inhibits prolactin release. Inducing dopamine receptors leads to insomnia/ sleep disturbances because they increase alertness
What are the effects of L-dopa drugs on sleep?
Causes –rarely- sleep disorder, but is associated with sudden onset of sleep (may be due to sleep deprivation and overexpression of GABAergic neurons)
What are the effects of antipsychotic drugs on sleep?
Antipsychotic, 1st generation block only D2, while 2nd generation block both D1 and D2, their effect on D2 of the mesolimbic and nigrostriatal postsynaptic D2 receptors showing therapeutic and extrapyramidal side effects (PD-like). However, they may block the inhibitory (autoreceptor) D2 receptors in the ARAS, leading to insomnia
How is noradrenaline synthesised?
· Adrenaline is synthesised through the same reaction of DA
- Tyrosine (mainly obtained from diet)
- Phenylalanine (from the liver)
· Tyrosine is the precursor amino acid in catecholamines synthesis
· DA is synthesised from L-tyrosine in the cytoplasm of the neurons
· DA is then converted into noradrenaline (NA) by DA β-decarboxylase
· NA is then converted into AD through phenyl ethanolamine N-methyl transferase (PNMT)
· AD is then stored into vesicle through pumps:
· Proton ATPase pumps that facilitate H+ influx into the vesicle
Proton antiporter: vesicular monoamine transporter (VMAT) that works as dopamine/H+ exchanger
What is the activity of noradrenaline?
· Adrenergic neurons of locus coeruleus (LC) are silent during sleep and active during wakefulness, induced by light
· After synthesis, NA is stored in the vesicle of the neurons in LC
· Action potential induces calcium influx and hence NA
· NA binds to adrenergic receptors mainly α1 and β to induce wakefulness and vigilance
· NA binds to presynaptic/inhibitory α2-(auto)receptors
· From the cleft NA is:
· Transported from the cleft back into the presynaptic cell
· Diffused in the blood
· Metabolised by MAO into 3, 4- dihydroxyphenylglyco aldehyde (DOPGAL), which is further metabolised by COMT
· Other LC neurons include GABA, glutamate, cholinergic
LC neurons express receptors for GABA, orexin/hypocretin, acetylcholine, and NA
What are the effects of TCA drugs on sleep?
By inhibiting reuptake of noradrenaline, accumulation of noradrenaline happens in the synaptic cleft, inducing insomnia
What are the effects of SNRI drugs on sleep?
Used for depression mainly which also inhibit reuptake of noradrenaline, inducing insomnia
What are the effects of beta blockers and alpha 1 blocker on sleep?
Blocks beta and alpha receptors which blocks ARAS neurotransmitter so induces sleep. Blocking post synaptic alpha and beta receptors induce sleep because noradrenaline binds to adrenergic receptors to induce wakefulness
How do amphetamines affect sleep?
· They block the reuptake of monoamines, especially dopamine and noradrenaline transporters
· Therapeutically, they are used to treat ADHD and narcolepsy
E.g. cocaine induces wakefulness
How is serotonin synthesised?
· Serotonin is synthesised by converting tryptophan into 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase (TPH)
· 5-HTP is converted into 5-HT (Serotonin) by 5-HTP decarboxylase
· 5-HT is transported into vesicles by VMAT (as with DA and NE) in the serotonergic neurons of raphe nuclei
· Action potential induces calcium-dependent serotonin release
· 5-HT binds to serotonin receptors (mainly 5-HT3, 5-HT6, 5-HT7 and 5HT2A-C) in the postsynaptic neurons of cerebral cortex, amygdala, thalamus, preoptic and hypothalamic areas, raphe nuclei, locus coeruleus and pontine reticular formation
· 5-HT1B autoreceptor supress TPH and provide negative feedback
· From the cleft 5-HT is recycled by selective serotonin transporters (SERTs) back in the neuron where 5-HT:
· Is stored in a vesicle through VMAT
Is metabolized through by the monoamine oxidase (MAO) system
What is the activity of serotonin?
· Once synthesized, serotonin is packaged into synaptic vesicles in the presynaptic neuron terminals.
· Upon neuronal depolarization or action potential firing, these vesicles undergo exocytosis, releasing serotonin into the synaptic cleft.
· Serotonin then diffuses across the synaptic cleft and binds to serotonin receptors (5-HT) on the postsynaptic neuron or other target cells.
· After exerting its effects, serotonin is removed from the synaptic cleft to prevent excessive activation of serotonin receptors.
· Serotonin reuptake is mediated by the serotonin transporter (SERT) located on the presynaptic neuron membrane, which transports serotonin back into the presynaptic neuron for recycling.
This process is crucial for maintaining appropriate serotonin levels and neurotransmission
What is the effect of 5-HT3 antagonists on sleep?
E.g. Ondansetron
Causes sleep as a side effect as it blocks ARAS serotonergic pathway, especially when given at high concentrations
What is the effects of SSRIs on sleep?
Used for depression, obsessive compulsive disorder and panic disorders, they block the reuptake of serotonin, therefore prevent sleep, inducing insomnia
What is the activity of acetylcholine?
· Cholinergic neurons from basal forebrain synthesised acetylcholine from acetyl CoA and choline by the enzyme choline acetyl transferase
· Released acetylcholine binds to postsynaptic muscarinic and nicotinic receptors:
· Ionotropic (ligand gated) ion channels: nicotinic receptors (N1 subdivision: peripheral, or N2 subdivision: central)
· Metabotropic (muscarinic G-protein coupled) receptors and there are 5 subtypes of these (M1-M5) M1, 3 & 5 are primarily excitatory, M2 & 4 are primarily inhibitory
· Acetylcholine is enzymatically hydrolysed by acetylcholinesterase into choline and acetate
Choline is recycled by presynaptic choline transporter to be used again for the biosynthesis of acetylcholine
How do anticholinergic drugs affect sleep?
Blocks muscarinic receptors which disrupts the muscarinic way of ARAS pathway so induces sleep
How does rivastigmine affect sleep?
Inhibits and blocks acetylcholinesterase so more Ach available, causing insomnia
How is Histamine synthesised?
· Histaminergic neurons are localised solely in the tuberomammillary nucleus of the posterior hypothalamus, and they project to various regions of the brain, while receiving inputs from LC and Suprachiasmatic Nuclei (SCN)
Histamine is synthesised in the cytoplasm through the conversion of amino acid histidine into histamine by histidine decarboxylase (HDC)
What is the activity of histamine?
Histamine released from the vesicle to bind to:
· Postsynaptic receptors (H1 and H2): GPCRs mostly excite neurons or potentiate excitatory inputs
Auto-receptor (H3) the provides negative feedback to reduce histamine synthesis and release, and regulate the release of other neurotransmitters (through H3 heteroreceptors on presynaptic neurons) such as dopamine, acetylcholine, noradrenaline, glutamate, and GABA
How do 1st generation antihistamines affect sleep?
1st generation antihistamines are sedative,
They exert their sedative effects by antagonizing histamine receptors in the brain, particularly H1 receptors. Histamine is a neurotransmitter that plays a role in wakefulness and arousal, so by blocking its action, these antihistamines promote drowsiness and sleepiness
How do other antihistamines affect sleep?
Other non-sedating antihistamines such as loratadine cause less sedation and psychomotor impairment than the older antihistamines, but can still occur; sedation is generally minimal
How is Orexin synthesised?
· Orexin-A and orexin-B (hypocretin-1 and hypocretin-2) are synthesised by a cluster of neurons in the perifornical area in the posterior lateral hypothalamus
· The orexin-producing neurons also synthesize glutamate and the inhibitory neuropeptide dynorphin
Orexin neurons project to all wakefulness and arousal brain regions
What is the activity of Orexin?
· Once released, orexin neuropeptides selectively excite and depolarise target neurons via two distinct GPCRs:
· OX1R: mainly expressed in LC, and of higher affinity to orexin-A (~100-fold) than orexin-B
· OX2R: mainly expressed in TMN, and of similar affinity for both orexin-A and orexin-B
Other regions (BF, VTA, raphe nuclei, and cortex) express both receptors
How do orexigenic drugs affect sleep?
Orexin receptor antagonists block the ARAS neurons, which induces sleep
What is the sleep pathway?
· The hypothalamus contains sleep-promoting neuronal populations: the ventrolateral preoptic area (VLPO) and median preoptic area (MNPO)
· VLPO and MNPO primarily express the inhibitory neurotransmitters GABA and galanin, and project to the wake-promoting system
· Adenosine has been shown to accumulate in VLPO
· There are four adenosine receptors: A1R, A2AR, A2BR, and A3R
· Adenosine and activate adenosine receptors (A2AR) of VLPO and hence activating inhibitor neurons that inhibit ascending pathway
· Moreover, adenosine activate the inhibitory receptor A1 expressed on ascending arousal areas such as BF
· Caffeine is adenosine receptor antagonist
VLPO receives signalling inputs from suprachiasmatic nucleus
What is the purpose of the suprachiasmatic nucleus?
A bilateral structure in the anterior part of the hypothalamus that regulates circadian rhythms in the body and promotes wakefulness. SCN receives many neuronal inputs, with the major inputs come from:
· Photic retinal projections: glutamatergic retinohypothalamic tract (RHT), and geniculohypothalamic tract (GHT) of neuropeptide Y (NPY), which activates SCN directly and indirectly by inhibiting presynaptic GABAergic neurons
· Raphe nuclei: release serotonin to the core of the SCN to potentiate glutamate input, and by inhibiting the inhibitory inputs
SCN contributes to innervating numerous brain areas such as:
· Hypothalamic and thalamic nuclei including VLPO, TMN, perifornical area
Pineal gland, where SCN releases noradrenaline to pinealocytes’ α1 and β1 receptors, triggering melatonin synthesis and release
What is melatonin?
· Melatonin (5 methoxy-N-acetyltryptamine) synthesis and secretion is enhanced by darkness and inhibited by light in response to photic information transmitted from the retina to SCN
· Nearly 80% of the melatonin is synthesised at night, reaches a peak in the middle of the night (between 2 and 4 in the morning) and decreases gradually during the second half of the night
· Melatonin activates high affinity ML1 sites and low affinity ML2 sites
· ML1 receptors: GPCR that inhibits adenylate cyclase in target cells (of SCN), suppressing SCN stimulatory signaling
· ML2 receptors (MT3), leads to phospho-inositides hydrolysis, inducing circadian rhythm phase shift
Melatonin receptor stimulator: Ramelteon (Rozerem)
How is insomnia treated?
· Insomnia treated by benzodiazepines or z-drugs (short-term ˂ 3 months)
· BDZ and z-drugs increase the efficiency of GABAergic pathways, by increasing the frequency of channel opening
· Z-drugs: Zolpidem, zopiclone, and zaleplon are non-BDZ: similar mechanism and different structure
· GABA receptors are:
1. Fast acting ionotropic GABA-A receptor
· Receptor activation requires the simultaneous binding of two GABA molecules to the receptor, one to each of the two binding sites at the interface of the α and β subunits
2. Slow-acting metabotropic GABA-B receptors: G-protein couple receptors that activate potassium channels to mediate the efflux of potassium and hence inhibiting the cell excitation (autoreceptors)
3. Fast-acting ionotropic GABA-C receptors
The GABAA and GABAC receptors are Cl– channels that mediate fast synaptic inhibition
What are the two main neurotransmitters in the CNS?
· Glutamate (excitatory)
· GABA (inhibitory)
Inhibitory is more common to aid suppression of the excitatory and prevent excessive stimulation
What is Epilepsy?
· Abnormal electrical activity in the brain
An imbalance in the excitatory and inhibitory neurotransmitters in the CNS
What are the 2 main categories of epilepsy?
GENERALISED:
· Absences
· Tonic-clonic (grand mal)
· Myoclonic (juvenile myoclonic epilepsy)
· Tonic
· Akinetic
FOCAL:
· Aware seizures (simple)
· Impaired awareness (complex)
Focal to bilateral tonic-clonic (secondary generalised)
Why does risk of epilepsy increase with age?
· Loss of neurons as age increases
· Risk of stroke increases, which can affect brain function
· Changes in brain structures
· Epigenetic factors
Infections over time
What are the main causes of epilepsy?
Vascular (stroke)
Infective
Neoplasm (cancer/tumour)
Drug (toxin/poison related, phenothiazines, benzodiazepine withdrawal, antibiotics, antiepileptics, alcohol, tramadol, antidepressants, ciclosporin, cocaine, lithium, barbiturates)
Inflammatory
Congenital (genetic polymorphisms)
Autoimmune
Trauma
Endocrine
Degenerative
Idiopathic (cause not always found)
Psychogenic
what are the differential diagnoses of epilepsy?
· Syncope
· Hyperventilation
· Migraines
· Panic attack
· Non- epileptic attack
· Transient global amnesia
Transient ischaemic attack
What investigations have to be done to diagnose?
· History/ collateral history
· Lying and standing BP
· ECG
· U&Es, bone profile
· Magnesium
· FBC
· MRI/CT
EEG
What are the response rates for treatment?
· 50% remit with one anticonvulsant
· A third of the remainder with 2 anticonvulsants
10% of the remainder remit with a third anticonvulsant
What are absence seizures?
· Developmental abnormality
· Childhood onset (4-8 years)
· Stare and cease normal activity for few seconds
· Characteristic, open, glassy staring eyes
· Turn pale and drop whatever in hand
· Quivering eyelids
· Relaxation of facial muscle causes typical absent impression on face
· Can lose consciousness which can be severe, but may be moderate or mild and therefore difficult to detect seizure
· Patients can experience amnesia, motoric arrest, hypolocomotion
· Complex forms can have automatisms such as walking, swimming, writing if consciousness sufficiently impaired. Lip licking, smacking, or mute mouth movements. Scratching, fumbling with clothes, other limb movements may occur.
Has characteristic 3Hz generalised spike on EEG
What are EEG?
Electroencephalography is a non-invasive diagnostic method used to record the electrical activity of the brain.
· Involves placing electrodes on the scalp using a special cap or adhesive patches. These electrodes detect electrical signals produced by the brain's neurons as they communicate with each other. The signals are then amplified and recorded by the EEG machine.
Measures the voltage fluctuations resulting from ionic current flows within the neurons of the brain. The recorded data shows these fluctuations as wave patterns, which are categorized based on their frequency (Hz) and amplitude (μV). Commonly identified wave types include alpha, beta, theta, and delta waves, each associated with different states of brain activity and consciousness
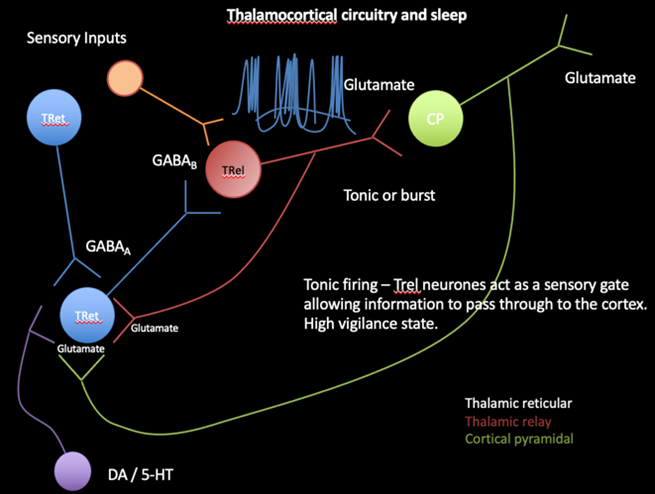
What is the thalamocortical circuitry?
1. Once sensory input is noted by the Tret neurons, this depolarises the TRel neurons through the action of GABA, binding to GABAa or GABAb neurons on the Trel
· TRel neurons are responsible for transmitting sensory information from the thalamus to the cortex (cortical pyramidal neurons) through glutamate. They work in either tonic or burst mode
· In tonic mode, the GABA binds to GABAb receptors, leading to slower inhibition causing TRel neurons fire regularly, acting as a sensory gate that allows information to pass through to the cortex. This is associated with a high vigilance state, meaning the brain is alert and able to process sensory information effectively.
· In burst mode, GABAa receptors are activated, causing TRel neurons fire in bursts, which is typically associated with lower states of vigilance, such as sleep.
2. The CP are the primary excitatory neurons in the cortex. They receive glutamate from TRel neurons which activates it and also send feedback to the thalamus, creating a loop of information flow
3. Sending glutamate to the Tret neurons, it keeps the cycle going
4. Dopaminergic (DA) and serotonergic (5-HT) neurons, provide modulatory input to both the thalamic and cortical neurons.
These neurotransmitters influence the overall activity of the circuitry, affecting states of arousal, mood, and sleep-wake cycles
How are calcium channels involved in this pathway?
· Involved in switching from burst to tonic firing in the Trel neurons when appropriate
· L-type calcium channels are high voltage activated channels (burst)
· T-type calcium channels are low voltage activated channels (tonic)
· In L-type calcium channels, a higher threshold of depolarisation is needed, leading to the slow depolarisation
· When high levels of calcium are recognised by the repetitive action potentials, body needs to control levels:
- apoptosis
- calcium-dependent potassium ion channels (potassium leaves the cell when calcium levels are too high, causing neurons to depolarise)
- channel inactivation- channel is switched off until next hyperpolarisation
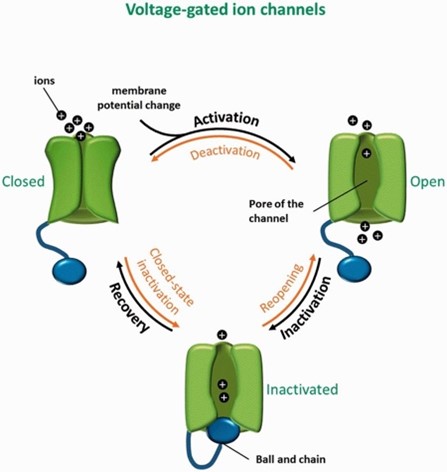
What is the switch from burst to tonic firing caused by?
INTRINSIC FACTORS:
· Changes in calcium build up and apoptosis
EXTRINSIC FACTORS:
Sensory input
How can activity in a single neuron be measured?
· Putting an electrode in the Trel
Measure action potential firing
How are absence seizures caused in the thalamocortical circuity?
· GABAb activation can promote absence seizure formation.
T-type channels require long periods of hyperpolarisation to be reactivated, the polymorphisms cause seizures
What are the treatment options for epilepsy?
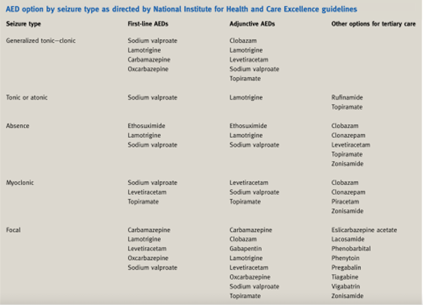
What is Generalised tonic-clonic seizures?
· Tonic phase- rigidity
· Followed by clonic phase
- convulsions
- frothing at the mouth
- tongue biting
- incontinence
· Drowsiness
· Unconsciousness
Coma
What is the pathophysiology of Generalised tonic-clonic seizures?
INITIATION PHASE:
· Abnormal Electrical Activity: GTCS often begin with abnormal, excessive electrical discharges in a specific area of the brain. This can be triggered by factors such as genetic predisposition, brain injury, or metabolic disturbances.
· Focus Spread: The abnormal electrical activity spreads from its initial focus to both hemispheres of the brain via neural pathways like the corpus callosum and subcortical structures.
TONIC PHASE:
· Sudden Onset: The tonic phase is characterized by a sudden loss of consciousness and a generalized increase in muscle tone.
· Sustained Muscle Contractions: Neurons in both hemispheres fire rapidly and synchronously, causing sustained muscle contractions. This leads to the characteristic rigidity seen in the tonic phase.
· Autonomic Changes: There may be an increase in autonomic nervous system activity, resulting in elevated blood pressure, heart rate, and sometimes pupillary dilation.
CLONIC PHASE:
· Rhythmic Jerking: Following the tonic phase, the clonic phase involves rhythmic, jerking muscle movements. This occurs due to alternating contraction and relaxation of muscles.
· Inhibitory Mechanisms: The clonic phase reflects a struggle between the excitatory activity causing muscle contractions and the brain's inhibitory mechanisms attempting to regain control and stop the excessive firing.
POSTICTAL PHASE:
· Cessation of Seizure Activity: The seizure eventually subsides as the abnormal neuronal firing diminishes.
· Residual Inhibition: After the seizure, there is often a period of residual neuronal inhibition, resulting in a postictal state characterized by confusion, drowsiness, and fatigue.
Recovery: The brain gradually returns to its normal state, and the individual regains consciousness, although they may not remember the seizure and may experience a headache or muscle soreness
What mutations increase the chance of tonic-clonic seizures?
Mutations in SCN1A and SCN1B disrupt the normal function of sodium channels in neurons, leading to an imbalance between excitatory and inhibitory signalling in the brain. This imbalance results in increased neuronal excitability and the propensity for seizures.
SCN1A mutations primarily affect inhibitory interneurons, reducing their ability to control excitatory neurons, while SCN1B mutations can affect the overall function and modulation of sodium channels
What are focal seizures?
Originate in a specific area of the brain and can have varied manifestations depending on the region affected. Unlike generalized seizures, which involve both hemispheres of the brain from the onset, focal seizures start in a localized cortical area but can sometimes spread to other parts of the brain.
What are the symptoms?
· Temporal Lobe: Emotional disturbances, déjà vu, auditory hallucinations.
· Frontal Lobe: Motor manifestations, speech disturbances, sudden behavioral changes.
· Parietal Lobe: Sensory abnormalities, such as tingling or numbness.
Occipital Lobe: Visual disturbances, such as flashes of light or visual hallucinations.
When is the rectal route useful?
· When the patient is unable to use oral route due to nausea, trauma, oesophageal stricture, being post-operative or uncooperative
· When the drug is less suitable for oral route due to acid hydrolysis, enzymatic degradation, GI side effects and taste issues
For the treatment of local conditions: haemorrhoids, colitis, laxatives
What are the problems with rectal drug delivery?
· Strong aversion to use
· Slow and incomplete absorption
· Variation in absorbed dose
· Inter subject variability (between patients)
Intra-subject variability (between doses)
What are the features of the rectum?
· Hollow organ (usually empty)
· Flat surface
· 3 major valves
· Terminated by anus (circular sphincter)
· Divided into 2 parts (ampulla and anal canal)
3 main veins from the ampulla
- Superior haemorrhoidal vein (drains to hepatic portal vein)
- middle HV (drains to systemic circulation)
- inferior HV (drains to systemic circulation)
What is the epithelial structure of the anal canal?
· Unkeratinised squamous stratified epithelium (20 %)
Primarily protective in nature (not good for drug absorption)
What is the epithelial structure of the ampulla?
· Simple columnar epithelium (80%), one cell layer, no villi
Goblet cells, primarily absorptive in nature
What are the rectal factors in drug delivery?
· Rectal wall (thickness)
· Rectal fluid (mucous)
Motility (variable)
What are the types of rectal formulations?
· Liquids (enemas)
Semi-solids
What is the liquid rectal formulations?
· Aqueous or oily solutions or suspensions for rectal administration
· High volume (100mL); Retention enema x-ray contrast media (Barium SO4); Treatments for inflammatory bowel disease; Laxatives (phosphate enemas)
· Micro-enema ( 3-5 mL); Laxatives (e.g. sodium picosulfate), for grand-mal seizures (e.g. diazepam) – antiepileptic.
Insert higher up rectum and warm before inserting tube
What is the semi-solid rectal formulations?
· Local therapy
· Cream
· Ointments
· Gels
· Foams- high surface area, useful in IBD
Solids (suppositories)
What are the components of suppositories?
· API
Base (usually fatty)- solid at RT, melts at body temperature e.g. Theobroma oil/ witespol
How are suppositories formulated?
· First need to melt the base
· Drug is then milled into small particles and incorporated as a fine powder into the melted base.
· The base (with drug) is then allowed to set in mould or final packaging.
Mould size is defined by volume of (usually fatty base) to give the final required weight. Therefore need to do a ‘displacement’ calculation to find the volume of base displaced by added ingredient(s)
You have been asked to supply some Lidocaine suppositories using an oily base (‘Witespol’) for a patient. The suppositories will be made using a 4g mould and each suppository will contain 10mg Lidocaine. You need to supply 50 suppositories. Given that the displacement value of Lidocaine in the oily base is 0.5, calculate how much base and active ingredient are required if a surplus of 5 suppositories are to be made

What are the different types of suppository bases?
WATER SOLUBLE BASE:
· Not used as much except glycerol/ gelatine mixtures used for constipation/ insufficient fluid in rectum for dissolution
· Fluid is withdrawn from the mucosa which can cause irritation and a laxative effect
· Useful in hotter climates
GLYCERIDE BASES
· Theobroma oil: not used due to polymorphic behaviour, poor mould release, low softening temperature, chemical instability and price)
Witespol is more commonly used
How is suppository drug released and absorbed?
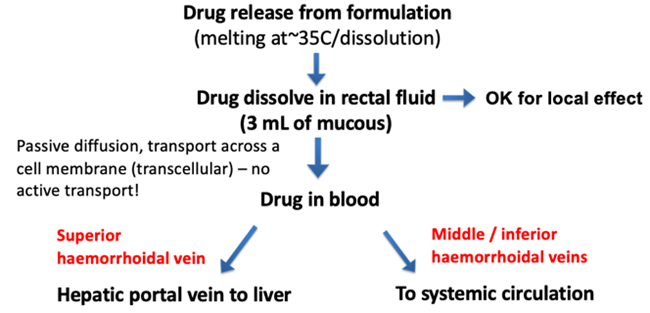
What drug properties affect absorption?
1. Solubility: Solubility in rectal fluid (3mL) determines concentration gradient at mucosa. Lipid solubility also required to partition into membrane
2. Surface properties: Relative to the surface properties of vehicle. Wetting of drug in suspension in base and in rectal fluid
Particle size: Particles < 100µm favourable, (milled) to prevent sedimentation in production. Also small size favourable for dissolution but too small (<45 µm) can agglomerate so ideal particle size is between 50-100 µm
What issues may arise with the base?
· Viscosity: affects sedimentation rate of drug particles
· Melting point: want a narrow melting range for rapid solidification after preparation, but wide enough for easy preparation
Compatibility: no major incompatibilities with the drug and allows for effective release of the drug
How should suppositories be inserted?
· If necessary, go to the toilet and empty bowels.
· Wash your hands.
· Remove any foil or plastic wrapping from the suppository.
· Either squat, or lie on your side with one leg straight and the other leg bent.
· Gently but firmly push the suppository, tapered end first, into the rectum (back passage). Push far enough so that it does not slip out.
· Close your legs and sit still for a few minutes. Avoid emptying the bowels for at least one hour (unless the suppository is a laxative).
Wash your hands again
What is the problem with rectal drug delivery?

Discuss why the rectal route would be suitable for the delivery of an acid-labile drug?
· Bypasses first pass metabolism where most of the drug would become metabolised and degraded
· A rich blood supply is present, facilitates rapid absorption
· Avoidance of food interference as some food and drink may interfere with oral drugs in the stomach
Rectal route has a more neutral pH compared to stomach so no degradation occurs
Epilepsy Therapeutics
What are the target areas for oral drug delivery?
· Local diseases: dental caries, periodontal disease, mouth ulcers, oral carcinomas, fungal infections
Systemic applications: avoid first pass metabolism, avoids stomach acid and inactivating enzymes
What are the main problems of drug delivery via the oral cavity?
· Patient acceptability: taste, mouth feel, odour
· Ease of absorption: normally only small amounts of drug absorbed from the mouth, needs to have the correct properties
· Retention: duration of application (mouthwashes less than a minute) and salivary washout
Distribution within oral cavity: drugs follow saliva when released, pool in lower part oral cavity and swallowed, not distributed throughout cavity
What are the different formulations available?
· Solutions: mouthwash
· Aerosol sprays
· Semi-solids: paste, ointment and gels
Solids: pastilles, lozenges, tablets and film
What are ointments?
Typical hydrophobic (liquid/white soft paraffin mix) bases, containing bio adhesive ‘sticky’ polymer particles - Orabase - sticky ointment that adheres to mouth ulcers (or holds in dentures)
What are gels?
Antimicrobial or soothing. Typical gel base (gelling agent such as carbomer dispersed in water, along with a preservative) with flavours, rapid release, tend not to be well retained
What are pastilles?
· The active is slowly released when chewed and allowed to dissolve in the mouth. Treats local conditions.
Typically the active ingredient contained in a base consisting of gelatine and glycerol or acacia and sucrose, along with a preservative, colour and flavour
What are lozenges?
· Dissolve in the mouth, sucked, active swallowed with saliva.
· Used to treat local infections of the mouth, pharynx, oesophagus. Lozenges may be moulded or compressed. usually contain the active ingredient in a suitable slow-release base, designed to give appropriate mouthfeel and dissolution properties
· No disintegrant is used because need to release drug slowly
What are sublingual tablets?
Similar to other oral drugs but tablet placed under the tongue to disintegrate and be rapidly absorbed through the oral mucosa e.g. GTN sublingual tablet
What are bio adhesive tablets?
Compressed tablets with adhesive that is retained on the oral mucosa, usually in the upper buccal pouch, and deliver drugs slowly either systemically into the mucosa or into the oral cavity. Do not contain DISINTEGRANT. Some of the polymers used in these buccal tablets are:
· Carbomers
· Hydroxypropylmethylcellulose
· Xanthan gum/ locust bean gum
chitosan
What are bio adhesive films?
Patches or films that adhere to the buccal mucosa and deliver drug for systemic absorption. They contain:
· active ingredient
· bio adhesive polymer
· film formation plasticisers
solvent
What is an important ingredient in buccal tablets?

Explain why drug delivery through the oral cavity is beneficial for systemic delivery of some drugs?
· Rapid absorption: The oral mucosa is highly vascularized, meaning it has a rich blood supply. Drugs administered via the oral cavity can be absorbed directly into the bloodstream through the mucous membranes, bypassing the digestive system and avoiding first-pass metabolism in the liver, leading to rapid onset of action
· Avoidance of gastrointestinal (GI) degradation: Some drugs are susceptible to degradation by stomach acid or enzymes in the gastrointestinal tract, which can reduce their bioavailability. By bypassing the GI tract, transmucosal delivery can protect drugs from degradation, leading to higher systemic concentrations and improved efficacy.
· Enhanced bioavailability: Transmucosal drug delivery can result in higher bioavailability compared to oral administration as drug is being absorbed directly into bloodstream
· Non-invasive route: Drug delivery through the oral cavity is non-invasive and relatively easy to administer, making it more convenient for patients compared to injections or other invasive routes of administration.
Versatility: The oral cavity offers multiple sites for drug delivery, including the buccal mucosa, sublingual mucosa, and gingival mucosa. This versatility allows for flexible dosing options and the possibility of sustained or controlled release formulations, depending on the specific requirements of the drug and the desired therapeutic outcome.
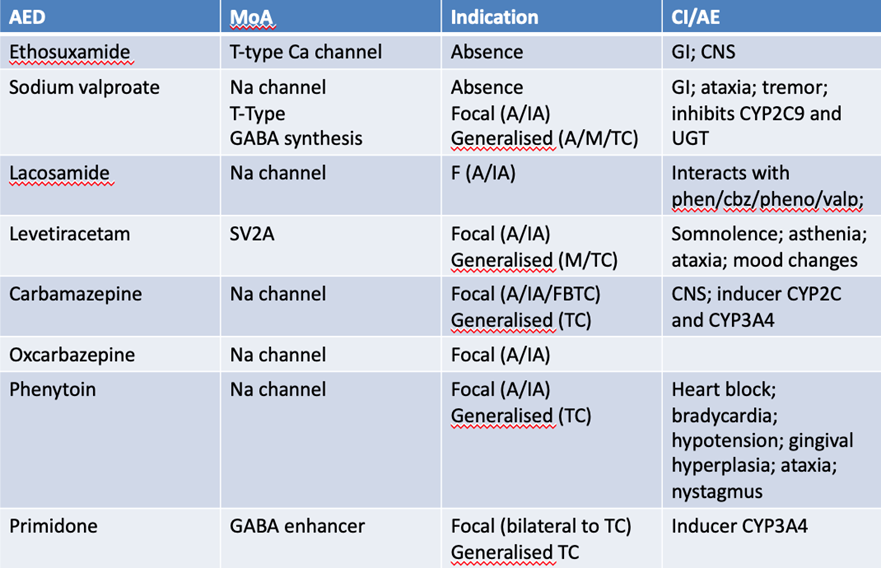
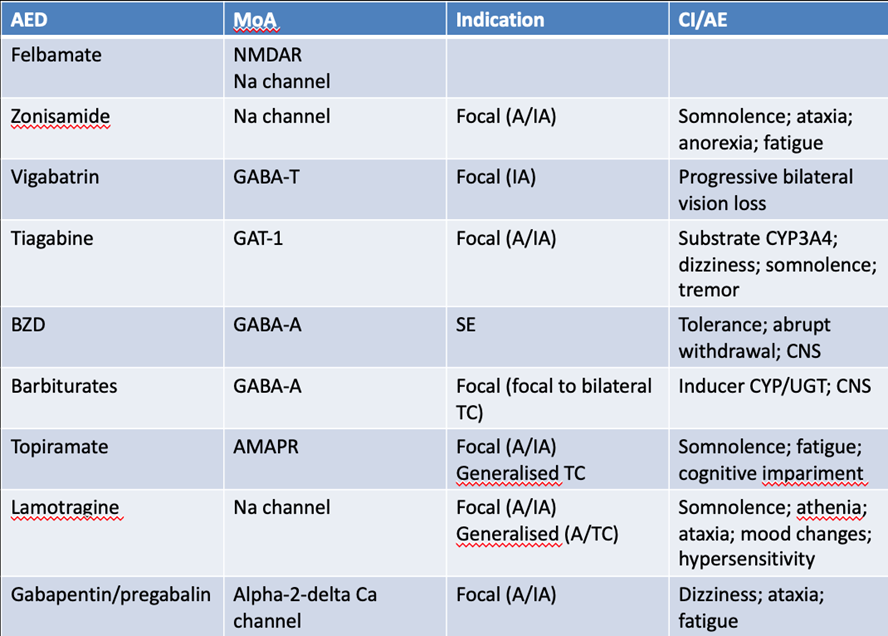
What are the current targets for antiepileptics?
· Sodium channel blockers: inhibiting firing of action potentials
- sodium valproate
- carbamazepine
- oxy carbamazepine
- lamotrigine (selective)
· Calcium channel inhibitors: L and T type both prevent sodium channel firing which prevents action potentials
- gabapentin/ pregabalin
- ethosuximide
· Enhancers/ inducers of GABA pathway
- clonazepam
- phenobarbitone
Inhibitors of the release of neurotransmitters
- levetiracetam
- phenytoin
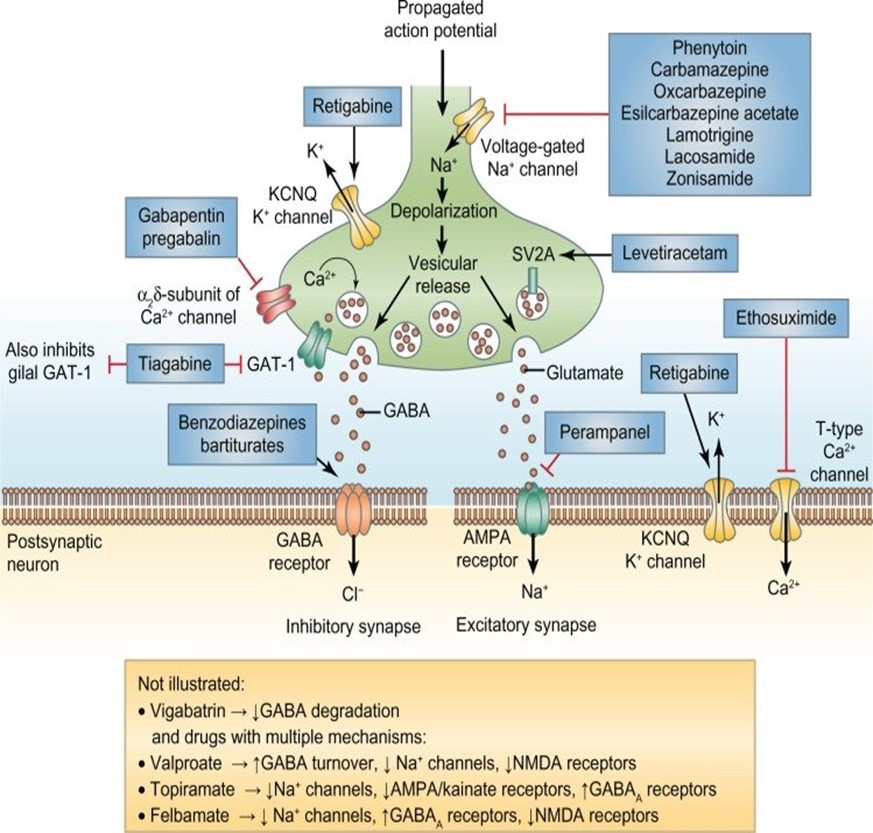
How can the NMDA pathway as a new target?
SOTICLESTAT (phase 3 trials)
· Inhibits specific enzyme CYP 46A1 which induces hydroxylation of cholesterol into 24HC
· The metabolite is a modulator of the NMDA pathway and increases excitatory glutamate signalling
· Preventing this conversion prevents the excitatory firing, preventing seizures
RADIPRODIL
· Subtype selective NMDA receptor antagonist
· Has preclinical efficacy in seizure models in rats
· Study terminated after insufficient enrolment of volunteers
AV101
Blocks and interferes with the kynurenine pathway quinolinic acid which activates NMDAR and exerts neurotoxic effects
How can the glutamate pathway as a new target?
JNJ-1813
· Glutamate is released in the synaptic cleft via calcium-dependent exocytosis, to bind:
· Excitatory postsynaptic ionotropic receptors (NMDA, AMPA or Kainate)
· Modulatory metabotropic receptors (mGluRs) that change the excitation rate of postsynaptic ion channels leading to either inhibition or excitation
· The mGlu2 receptor functions as a presynaptic autoreceptor that, upon activation, decreases the release of the excitatory neurotransmitter glutamate
· JNJ-1813 is another class of compounds, metabotropic glutamate receptor 2 (mGluR2) agonist (PAM: positive allosteric modulator)
Currently in phase 2 clinical trials
How can the potassium channel pathway as a new target?
XEN1101:
· Opens potassium channel and effluxes out positive charge
This reduces excitation of glutaminergic neurone
How can oligonucleotides be used as new targets?
STK-001
· First drug that modifies the disease
· Dravet syndrome is a severe and progressive genetic epilepsy characterized by frequent, prolonged and refractory seizures, beginning within the first year of life
· Dravet syndrome is difficult to treat and has a poor long-term prognosis
· One of the most common channelopathy affects the α1 subunit of a neuronal voltage‐gated sodium channel that is coded by the SCN1A gene (Chromosome 2q24.3)
· In approximately 85% of cases, DS is caused by spontaneous, heterozygous loss of function mutations in the SCN1A gene
· DS patients have two copies, one wild-type and the other is mutant
· STK-001 is an oligonucleotide that has the potential to be the first disease-modifying therapy to address the genetic cause of Dravet syndrome
· Oligonucleotides are short synthetic polymers of nucleotides that are complementary to specific regions of mutant –pre- mRNA
STK-001 is designed to upregulate NaV1.1 protein expression by leveraging the non-mutant (wild-type) copy of the SCN1A gene to restore physiological NaV1.1 levels, thereby reducing both occurrence of seizures and significant non-seizure comorbidities
How can the glycolysis pathway be used as new targets?
KETOGENIC DIET AND DRUGS
· A ketogenic diet should be applied under a specialist supervision and broadly consists of a low carbohydrate, high fat diet
· Ketogenic diet will lead to higher proportion of ketones in the bloodstream which seems to reduce the frequency of seizures
Treatment would usually begin with only one medicine, if this is ineffective, a different drug may be tried
Outline the 4 main mechanisms for current antiepileptic drugs and an example of each drug class and an alternative new therapeutic target and the name of the drug candidate
1. Sodium channel inhibitors: sodium valproate
2. Calcium channel inhibitors: pregabalin
3. GABA inducers: phenobarbital
4. Excitatory neurotransmitters inhibitors: levetiracetam
Alternative therapeutic target: oligonucleotide: STK-001
A 22-year-old student was prescribed diphenhydramine to treat her allergic rhinitis. Few days later she came to the pharmacy complaining from drowsiness, poor cognitive skills and lack of concentration. Explain the reasons behind these side effects
· 1st generation anti9HAVE ANTICHOLINERGIC EFFECT histamines which can cross the BBB
· Histamine is an inhibitor in ARAS system which is involved in sleep
· COGNITIVE EFFECT IS IMPPAIRED BY BLOCKING CHOLINERGIC PATHWAY