MCAT General Chemistry Review
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is ▲G (Gibbs Free Energy) and what does it mean if it is positive or negative?
When ▲G is positive, it means the reaction is nonspontaneous and NEEDS energy from outside to proceed. When ▲G is negative, it means the reaction is spontaneous and DOESN’T need energy from outside to proceed.
What is Activation Energy?
Activation Energy is the minimum amount of energy necessary for a reaction to take place.
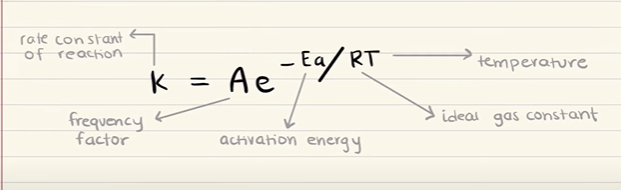
What are the relationships present in the Arrhenius equation?
A (the frequency factor) indicates how often the molecules collide in the right orientation. This can be increased by adding more molecules to the vessel.
Higher temperatures, higher A values and lower activation energy will INCREASE the rate of a reaction.
What factors affect the rate of reaction?
The greater the concentration of the reactants, the greater the rate of reaction.
The higher the temperature, the higher the rate of reaction (unless a very high temperature starts to denature the catalyst).
Catalysts such as enzymes can speed up reactions as they lower activation energy.
The medium used is important (polar/nonpolar, gas/liquid/solid). Using the correct one for the molecules being reacted will also speed up the reaction.
What is the rate law?

Steps to solving rate law problems (with experimental data).
Find two trials where the concentration of one reactant changes but the concentration of the other reactant stays the same.
See how the rate changes for that reactant and find the order of that reactant.
Repeat for the other reactant.
Plug in values from any trial to solve for k.
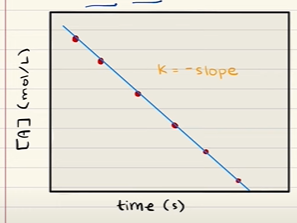
What is a zero order reaction?
A zero order reaction has a rate of formation of the product that is independent of the concentrations of the reactants. The only way to change the rate of such a reaction is to change the temperature (this will increase k) or add a catalyst. k= -slope
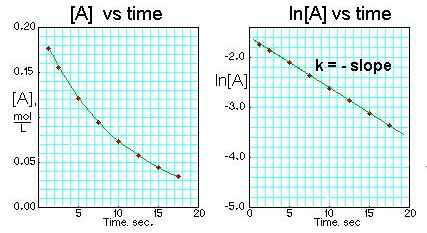
What is a first order reaction?
The rate of formation of the product is directly proportional to A SINGLE reactant. If the concentration of that reactant is doubled, the rate will also double.
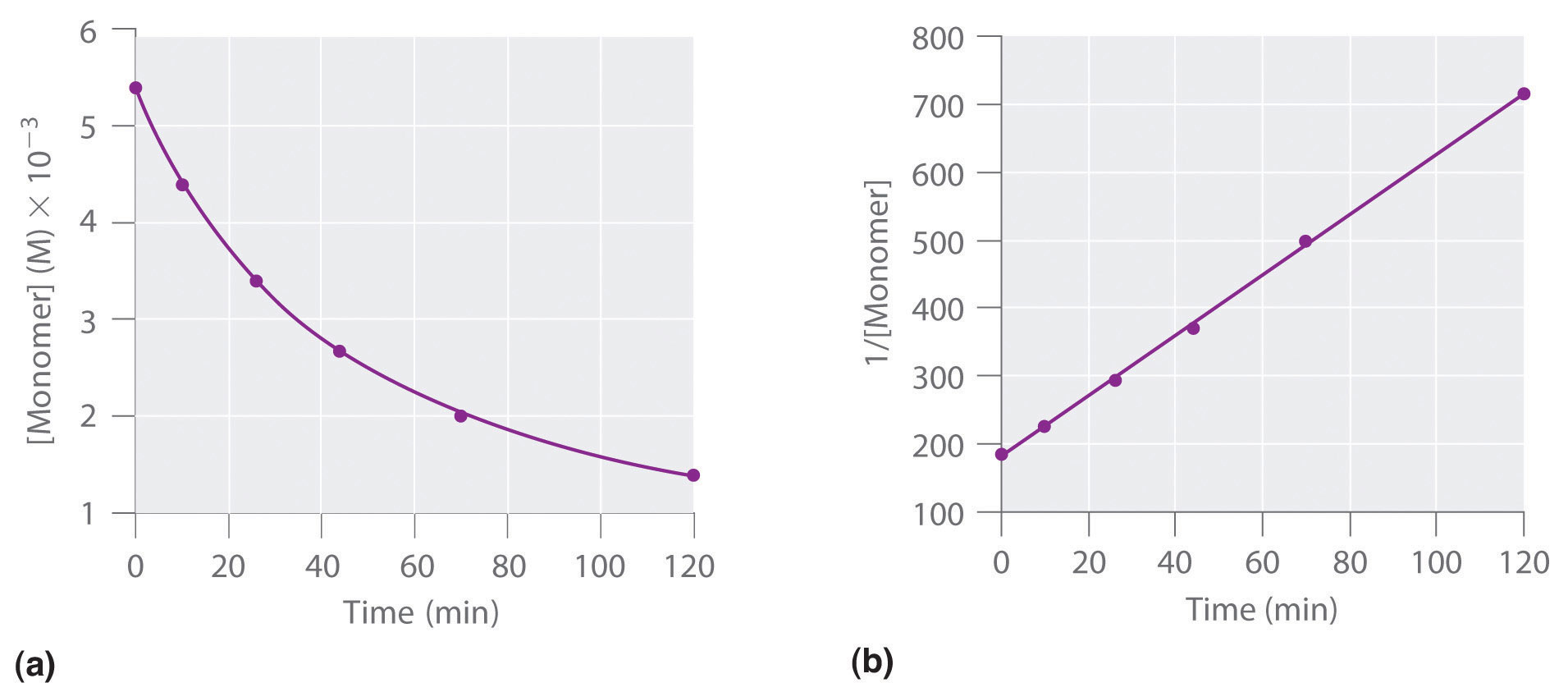
What is a second order reaction?
The rate of formation of the product is either directly proportional to the concentrations of two reactants OR proportional to the square of the concentration of a single reactant.
k=slope in an ln graph
What step determines the rate law for the reaction?
The slow step.
