Chemistry Final 🙂🔫
1/180
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
181 Terms
significant 0s
leading 0s are never significant
if a decimal is present, trailing 0s are significant
ex. 800 vs 800.
measurement
always must estimate one digit past that which you know
if + or - with different levels of precision, you must round so the answer has the same amount of decimal places as the number with the least
if x or / with different levels of precision, you must round so the answer has the amount of sig figs as the number with the least
precision
how consistent data is
accuracy
how close data is to accepted value
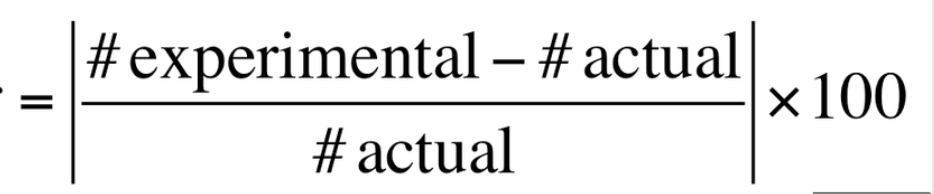
percent error
scientific notation
moving the decimal so #s are shorter (can be smaller or bigger)
_ x 10n
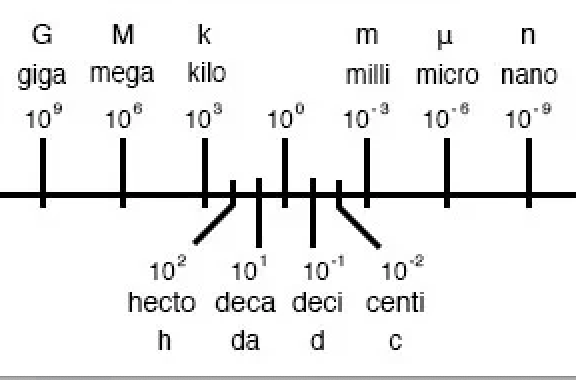
metric prefixes
dimensional analysis
used to convert among units
atom
consists of e- cloud with negatively charged e- that have negligible mass very far away from a nucleus
nucleus
positive protons + neutral neurons
p+ and n0 both have a mass of 1 amu
protons
identify element
positive
1 amu
neutrons
hold together nucleus
neutral
1 amu
electrons
participate in chemical reactions
negative
1/1840 amu
atomic number
# of protons (c)
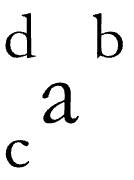
mass number
# of protons + # of neutrons (d)
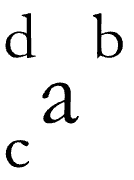
element symbol
identified by p+ (a)
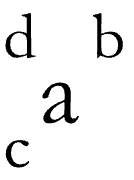
net charge
# of protons + # of electrons (b)
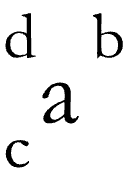
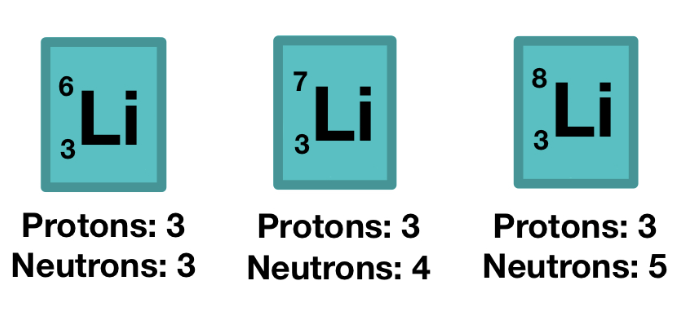
isotopes
same element, different masses
ions
same element, with net charge
losing e- = positive charge (cation)
gaining e- = negative charge (anion)
average atomic mass
weighted average of isotope masses
mole
a name for a #
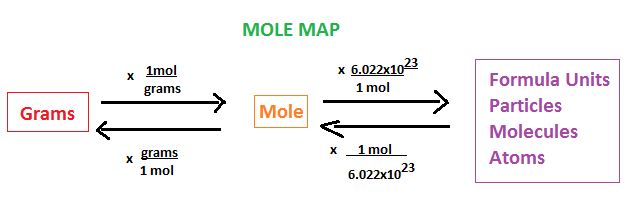
Avogadro’s number
# of things in a mole (6.022 × 1023)
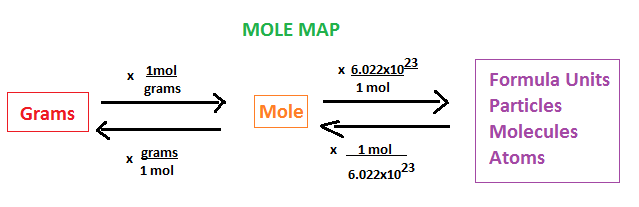
molar mass
the mass of one mole of a substance
waves
wavelength + frequency
low energy: long wavelength + low frequency
high energy: short wavelength + high frequency
Planck’s constant
6.626 × 10-34 Jxs
photoelectric effect
light can cause metals to release e-
ability to release e- depends on wavelength, not intensity of light
proves that ligh behaves as not only waves, but particles called photons
Bohr model
excited H atoms release specific wavelengths (not a full spectrum)
e- must exist only at specific energy levels
emission spectra makes sense only for H+
every element has a unique emission spectrum
e- absorbs photons of specific energy
rise to higher shell (“excited”) state
e- falls back to “ground” state
release photons of specific energy
Quantum Mechanical Model
e- exists in orbitals within s (1), p (3), d (5), & f (7) subshells
each orbital holds 2 e-
orbital diagrams
use to fill in the e-
s block (columns 1-2 + column 18, row 1) ← starts with 1s
p block (column s13-18 except row 1) ← starts with 20
d block (columns 3-12) ← starts with 3d
f block (lanthanides + actinides) ← starts with 4f
Aufbau principle
fill orbitals in order
Hund’s rule
don’t pair up until you have to
Pauli Exclusion principle
electrons in the same orbital need opposite spins
valence electrons
e- in the outermost shell
core electrons
not valence electrons (not in outermost shell)
electron configuration
starts with last term, then builds up from it
noble gas configuration
start with nearest previous group 8A element (noble gases), add e- that come after
electron configuration of ions
main group elements take valence configuration of nearest noble gas
atoms lost valence e- first (s and p)
The Periodic Table
developed by Mendeleev
organization:
groups (columns) & periods (rows)
metals, metalloids, and nonmetals
s & p block (main group), d block (transitional metals), f black (inner transitional metals)
Mendeleev
developed the Periodic Table
periodic law
elements grouped by similar, recurring properties (not order by mass)
Moseley
discovered the atomic #
metals
solid
ductile
metallic lustre
brittle
semi-conductors
*bottom left of Periodic Table*
metalloids
properties of both metals and nonmetals
metallic lustre
brittle
semi-conductors
*staircase down from B*
nonmetals
can be solid, liquid, and gas
dull
brittle
insulators
*top right of Periodic Table*
alkali metals
outer level electrons - 1
G1
very reactive
soft
silvery
shiny
low density
ex. Li, Na, K, Rb, Cs, Fr
alkaline earth metals
characterized by loss of 2 e-
G2
harder & denser (than alkali metals)
Be doesn’t react with water
Mg only reacts with steam
Ca, Sr, Ba, Ra react with water
ex. Be, Mg, K, Ca, Sr, Ra
halogens
G7
nonmetals (not conductive)
brittle & crumbly when solid
poisonous & smelly
ex. F, Cl, Br, I, As, Ts
noble gases
G8
stable
colorless
odorless
inert
ex. He, He, Ar, Kr, Xe, Rn
transitional metals
when ionizing, typically loses the 2s e- first
d block
good conductors
harder/denser with higher melting points
less reactive
inner transitional metals
f block
lanthanides
actinides
lanthanides
all similar in properties
shiny metals
similar reactivity of alkaline earth metals
elements 58-71
actinides
all radioactive
first 4 are found naturally, the rest are lab-made
elements 90-103
effective nuclear charge
change of p+ minus shielding effect by e- (Zeff)
when Zeff is higher, it’s harder to remove an e- because it’s being more attracted AND if Zeff is higher, the valence e- are pulled in more resulting in a smaller atomic radius
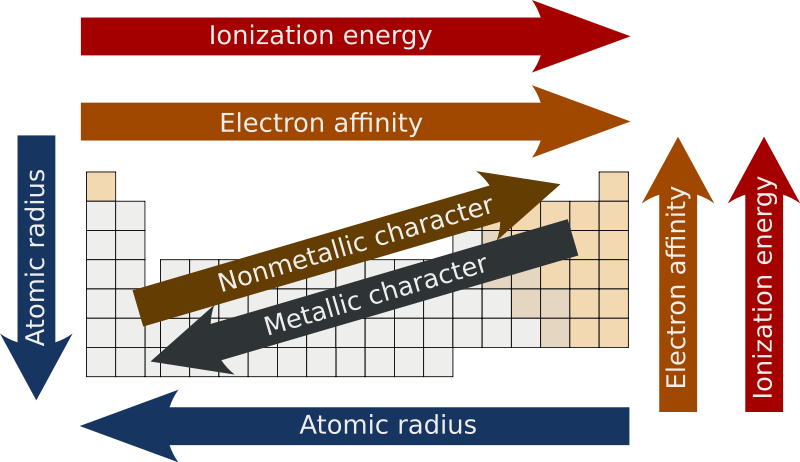
atomic radius
measure of the size of an atom
atoms get bigger with more shells & as the valence e- get further away they’re less attracted by the nucleus (Zeff also has a smaller effect)
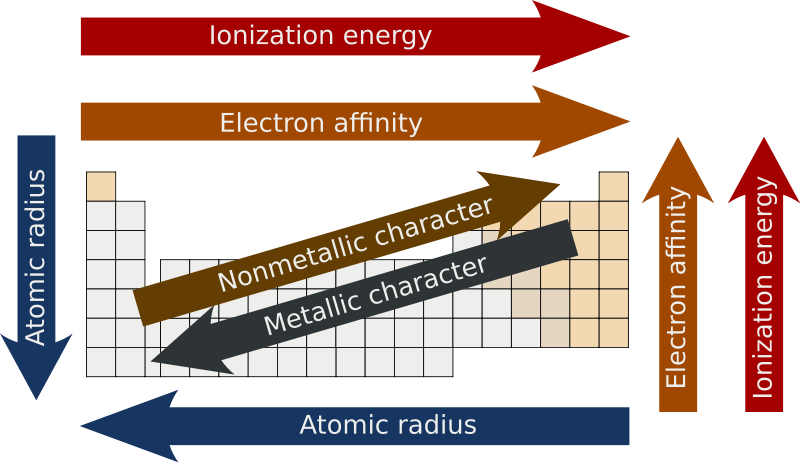
ionization energy
energy required to remove an e-
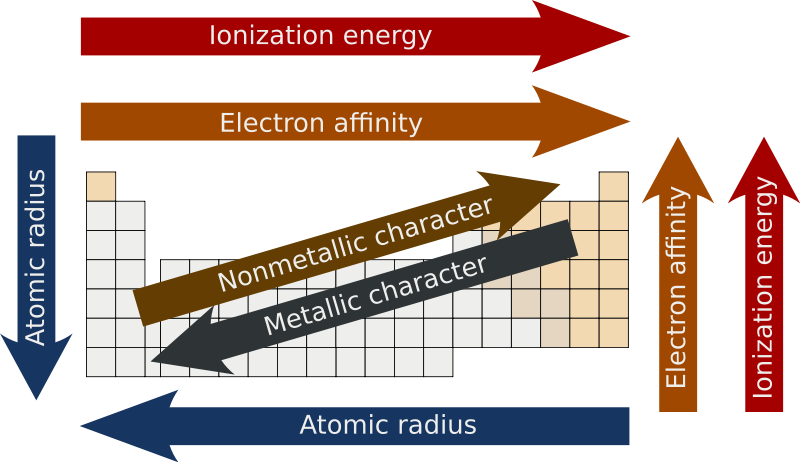
electronegativity
ability to attract e- in a chemical bond
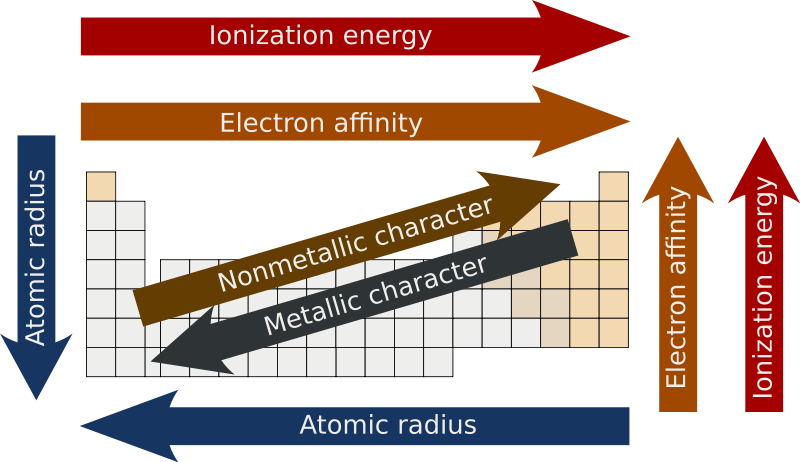
metallic character
trends with low ionization energy
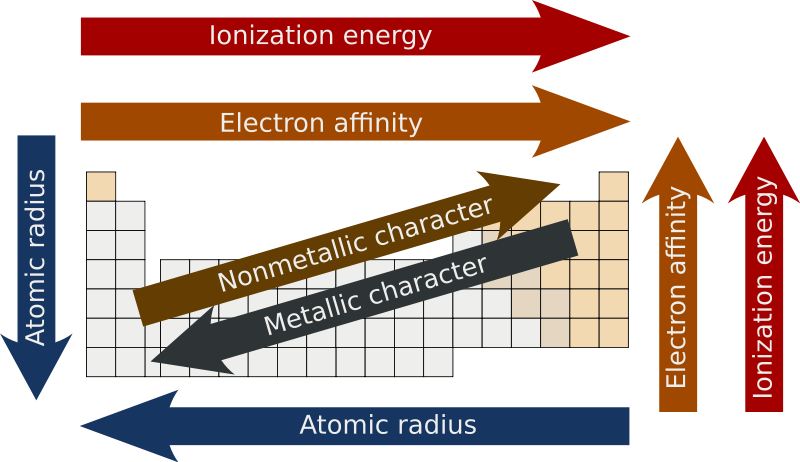
reactivity
most reactive metals have low ionization energy
most reactive nonmetals have high ionization energy
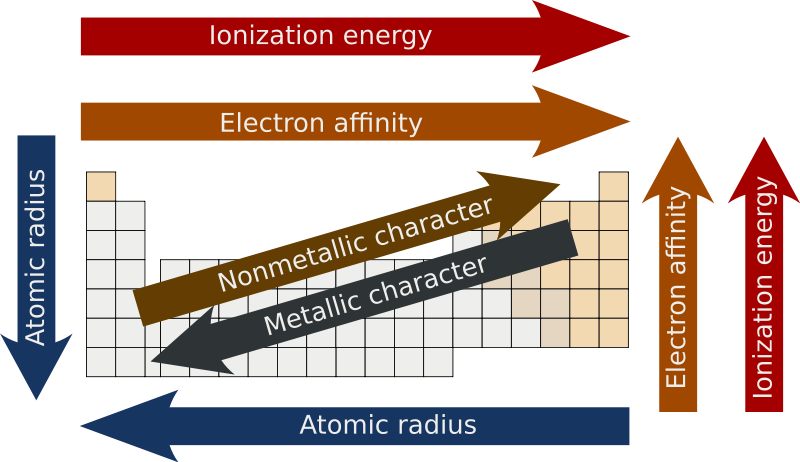
octet rule
atoms form bonds, needs 8 e- to satisfy it
duet for H+
ionic bonding
nonmetals (high electronegativity) pull electrons from metal (low ionization energy)
forms crystal lattice
covalent bonding
two nonmetals/metalloids (similar electronegativities) share e- to satisfy octet rule
metallic bonding
“sea” of delocalized valence e- amid metal cations
explains electrical conductivity & malleability of metals
network solids
huge covalent molecules with very high melting points
crystal lattice
“formula units” (not molecules), lattice energy
lattice energy
energy released when ions stick together
increase with increasing charge of ions
decreases with increasing size of ions
bond lengths
average distance between nuclei
bond strength
energy required to break
Lewis structure
line represents 2 shared e-
total bonding & nonbonding e- = sum of atoms valence e-
satisfy octets with lone pairs and/or double/triple bonds
resonance
resonance
when Lewis structures have alternating single-double bond pattern, equivalent structure can be drawn
geometry
valence shell e- pair (VSEPR) theory
e- repel, causing bonds & lone pairs to be as far apart as possible
steric number
# of atoms + lone pairs
single/double/triple bonds: each count for 1 group
electron pair geometry
3 options:
linear (180º)
trigonal planar (120º)
tetrahedral (109.5º)
molecular geometry
lone pairs cause different names
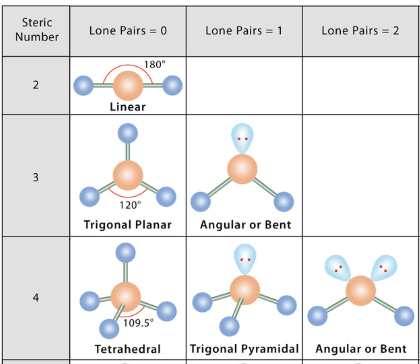
bond angles
if there are lone pairs, add “<“
bond polarity
from unequal sharing of e-
bond is polar if the electronegativity difference is >0.4
molecular polarity
from unequal sharing of e-
bond dipoles can “cancel” if symmetrical
net dipole moment
exists when molecule is asymmetrical (pos → neg)
for steric #s of 2-4, lone pair will always result in this
ionic compounds
metal + nonmetal
binary: 2 elements (-IDE ending)
ternary: at least 3 elements (-ITE, -ATE)
hydrogen adds an H+ to the formula, charge becomes less negative
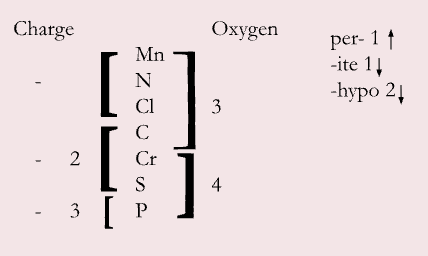
stock system
use Roman numerals to indicate charge of ion
can use with almost all d/f block + Sn + Pb
exceptions: Ag2+, Cd2+, Zn2+
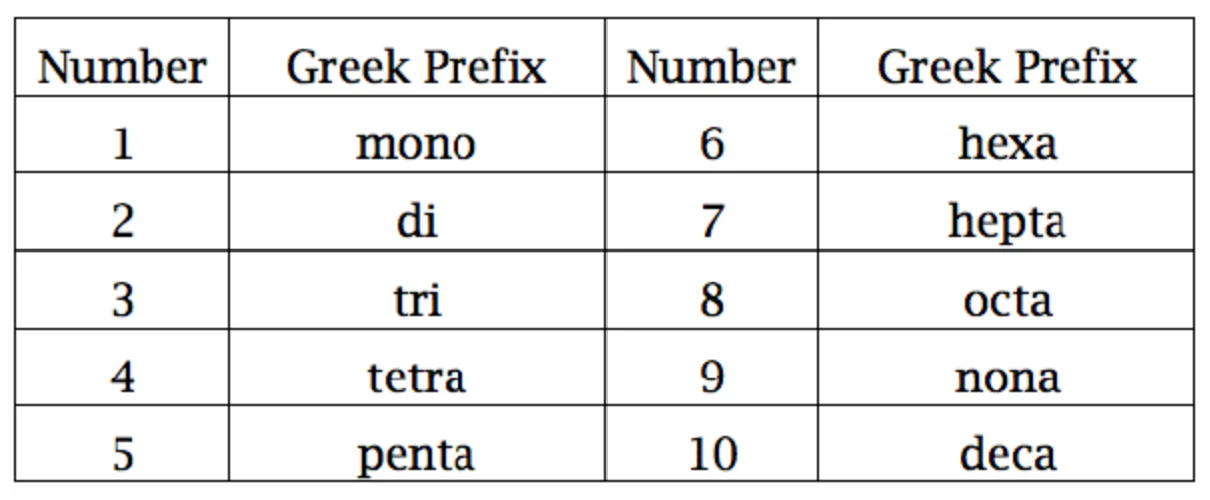
covalent compounds
two nonmetals/metalloids
when naming use a prefix for every atom except if the first atom only has one
molecular acids
H+ is always written first (except H2O)
binary: hydro __ic acid
ternary: H+ + polyatomic ion
-ITE → -OUS
-ATE → -IC
acid formula
formula begins with H+
made up of nonmetals
type of molecule compound (inorganic)
formula mass
sum of the average atomic masses of each element represented in the formula
amount of element x atomic mass of element (same things for any other elements, then add all of them together)
percent composition
part/whole x 100 (used as conversion factor)
hydrates
contains H2O in the compound, water + other molecules
weight of H2O: 18.02 g (leave as is for calculation)
empirical form
gives ration of elements in compound (ionic)(not actually #s of arrangement of atoms)
molecular form
actual formula of a compound (multiple of empirical formula)
chemical reaction
indicators
color change
temperature change
formation of gas or precipitate
balancing
only change coefficients, never subscripts
don’t balance until you’ve made the correct products
synthesis
one product
ex. A + B → C
decomposition
one reactant
ex. C → A + B
single replacement
element replaces element in compound
ex. AB + C → AC + B
double replacement
ions in two compounds swap partners
ex. AB + CD → AD + CB
combustion
hydrocarbon + O2 → CO2 + H2O
diatomics
consisting of two elements: Br, I, N, Cl, H, O, F
only elements can be this
activity series
specifically single replacement; can predict whether an element will react with an anion
if a metal/nonmetal is more active it’ll react w/ a less active ion
why reactions are non reversible
nonmetals activity series: F, C, O, Br, I, S, P
stoichiometry
based on the law of conservation of mass; the amount of products and reactants in a reaction
mole-mole, mole-mass, mass- mole, mass-mass, molecules, molecules, etc.
percent yield
actual/theoretical x 100
actual yield
might not end up with the exact amount of product you hoped for
theoretical yield
everything goes into 100
limiting reagent
whatever is less abundant/runs out first; prevents more of the molecule from being formed
the reagent in short supply limits the quantity of product that can be produced