Atomic Structure (copy)
1/51
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
Define ionic bonding
Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer
Do metal atoms form positive or negative ions?
Positive
Do non-metal ions form positive or negative ions?
Negative
When are ionic bonds stronger?
When the ions are smaller and/or have a higher charge
What structure do ionic crystals have?
Giant lattices of ions
Why are positive ions smaller compared to their atoms?
It has one less shell of electrons so the ratio of protons to electrons has increased so there is greater net force on remaining electrons holding them more closely
Why are negative ions formed from groups 5 to 7 larger than the corresponding atoms
The negative ion has more electrons than the corresponding atom but the same number of protons, so the pull of the nucleus is shared over more electrons and the attraction per electron is less, making the ion bigger.
Why does the size of ionic radius increase going down a group?
As one goes down the group the ions have more shells of electrons.
What is a covalent bond
A covalent bond is a shared pair of electrons
What are dative covalent bonds?
Forms when the shared pair of electrons in the covalent bond come from only one of the bonding atoms
What is metallic bonding
The electrostatic force between the positive metal ions and the delocalised electrons
3 main factors affecting the strength of metallic bonding
1- more protons = stronger bond
2- more delocalised electrons = stronger bond
3-smaller ion = stronger bond
What are simple molecular structures
Covalently bonded molecules with intermolecular forces (van der waals, permanent dipoles, hydrogen bonds) between them.
Describe the boiling/ melting points of ionic structures
High boiling/ melting points of giant lattice of ions with strong electrostatic forces between oppositely charged ions.
Describe the solubility in water of ionic structures
Generally good
Describe the conductivity when solid of an ionic sturcture
Poor - ions can’t move/ fixed in lattice
Describe the conductivity when molten of ionic structures
Good as ions can move and carry charge
Describe the boiling/ melting points of simple molecular structures
Low because of weak intermolecular forces between molecules (van der waals, hydrogen bonds, permanent dipoles)
Describe solubility in water of simple molecular structures
Generally poor
Describe the conductivity when solid of simple molecular structures
Poor as no ions to conduct and electrons are localized (fixed in place)
Describe the conductivity when solid of simple molecular structures
Poor as no ions to carry charge
Describe the boiling/ melting points of metallic sturcture
High as strong electrostatic forces between positively charged ions and sea of delocalised electrons
Describe the solubility in water of metallic structures
Insoluble
Describe the conductivity when solid of metallic structures
Good as delocalized electrons can carry charge and move throughout structure
Describe the conductivity when molten of metallic structures
Good
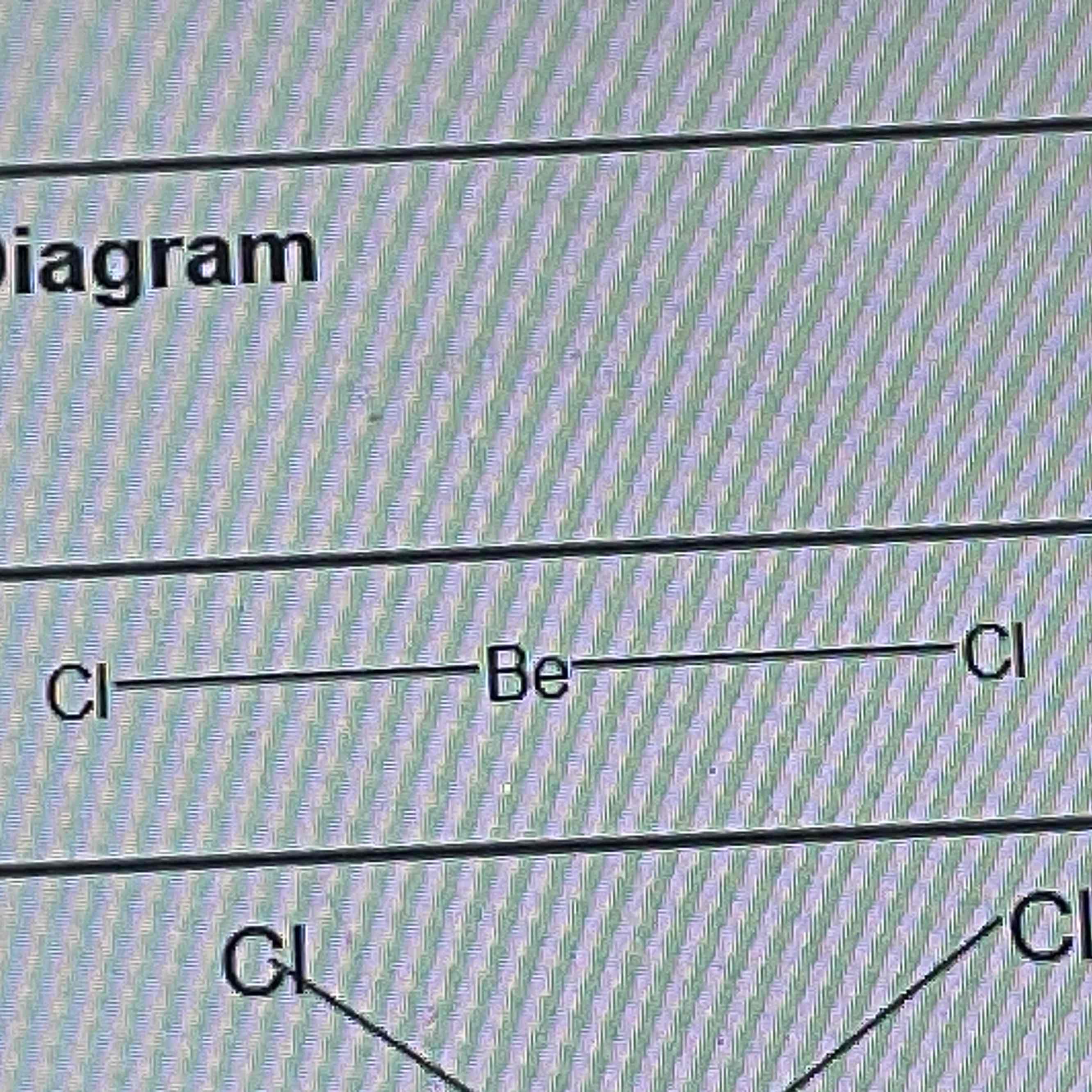
linear
No of bonding pairs = 2
No of lone pairs = 0
Bond angle = 180º
Examples = CO2, CS2, HCN, BeF2
trigonal planar
No of bonding pairs = 3
No of lone pairs = 0
Bond angle = 120º
Examples = BF3, AlCl3, SO3, NO3[-] , CO3[2-]
![<p>No of bonding pairs = 3</p><p>No of lone pairs = 0</p><p>Bond angle = 120º</p><p>Examples = BF3, AlCl3, SO3, NO3[-] , CO3[2-]</p>](https://knowt-user-attachments.s3.amazonaws.com/0bcdc76b-1cf4-4a7d-992f-845370213399.jpeg)
tetrahedral
No of bond pairs = 4
No of lone pars = 0
Bond angle = 109.5º
Examples = SiCl4, SO4[2-], ClO4[-], NH4[+]
![<p>No of bond pairs = 4</p><p>No of lone pars = 0</p><p>Bond angle = 109.5º</p><p>Examples = SiCl4, SO4[2-], ClO4[-], NH4[+]</p>](https://knowt-user-attachments.s3.amazonaws.com/85ef83b5-daf1-42a8-a8ab-3641c1596e04.jpeg)
trigonal pyramidal
No of bond pairs = 3
No of lone pairs = 1
Bond angles = 107
Examples = NCl3, PF3, ClO3, H3O[+]
![<p>No of bond pairs = 3</p><p>No of lone pairs = 1</p><p>Bond angles = 107</p><p>Examples = NCl3, PF3, ClO3, H3O[+]</p>](https://knowt-user-attachments.s3.amazonaws.com/57b83f59-1770-4a38-81a7-2c189fb606ff.jpeg)
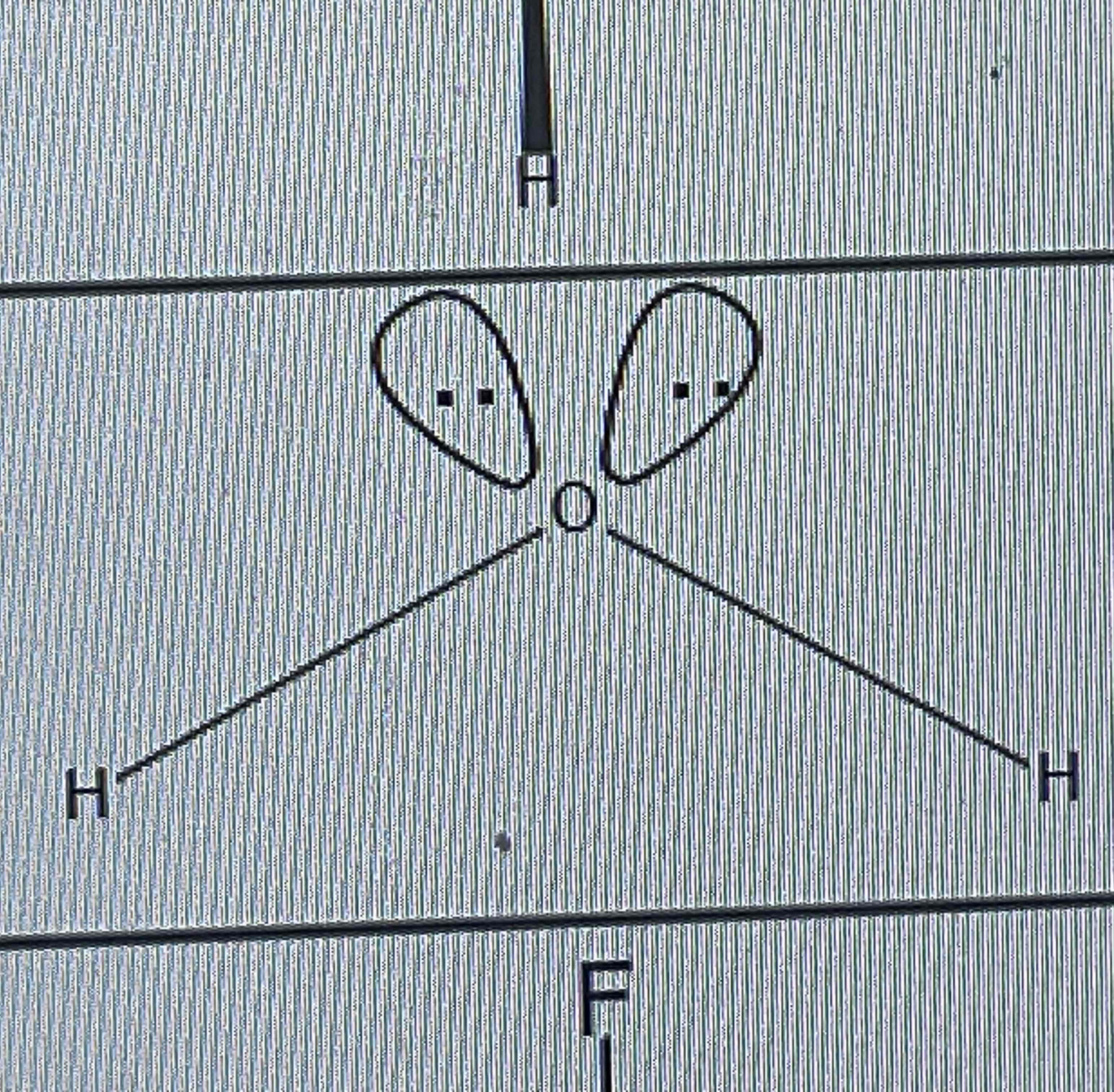
bent
No of bond pairs= 2
No of lone pairs = 2
Bond angle = 104.5º
Examples = H2O, H2S, OF2, SCL2
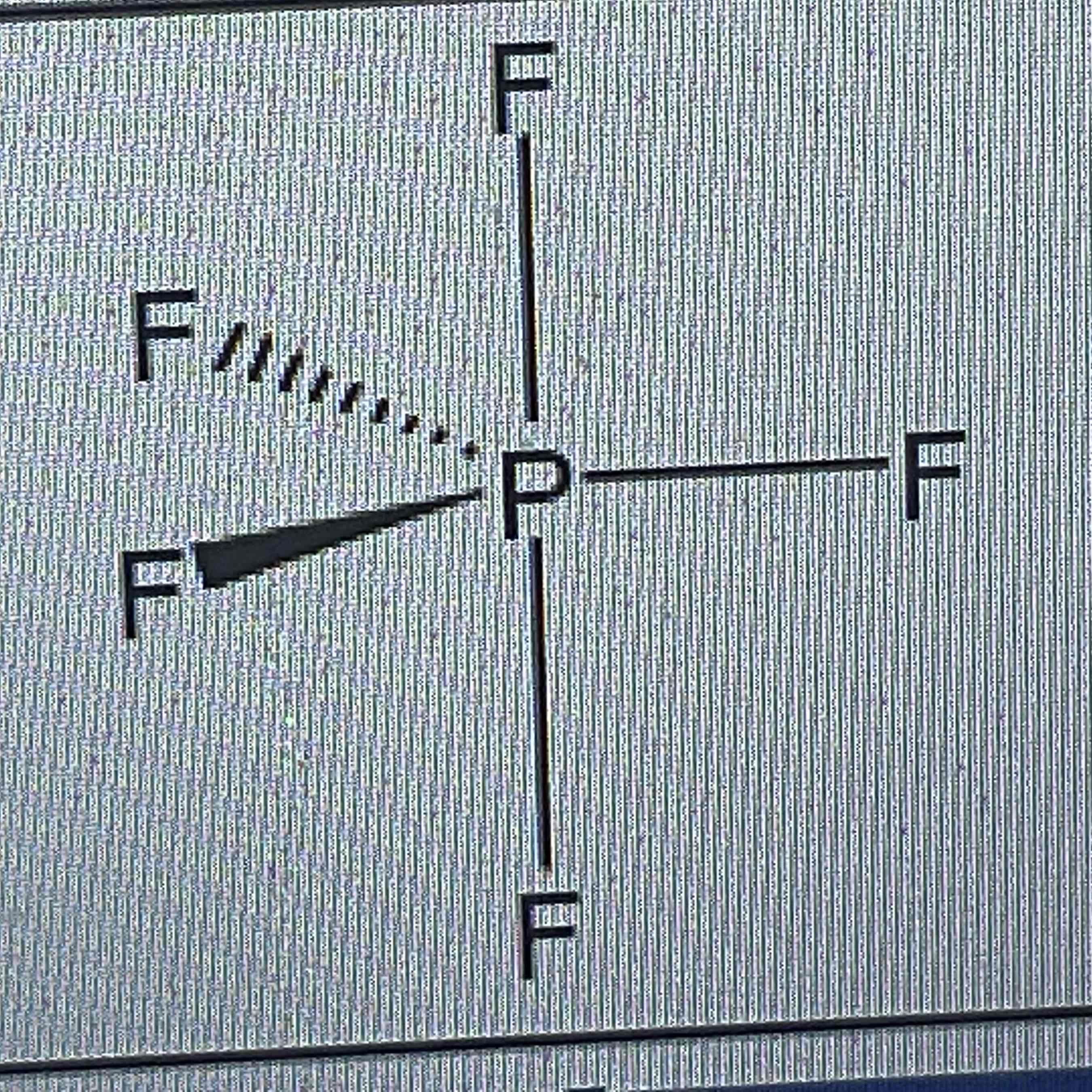
trigonal bipyramidal
No of bond pairs = 5
No of lone pairs = 0
Bond angles = 90º and 120º
Example= PCl5
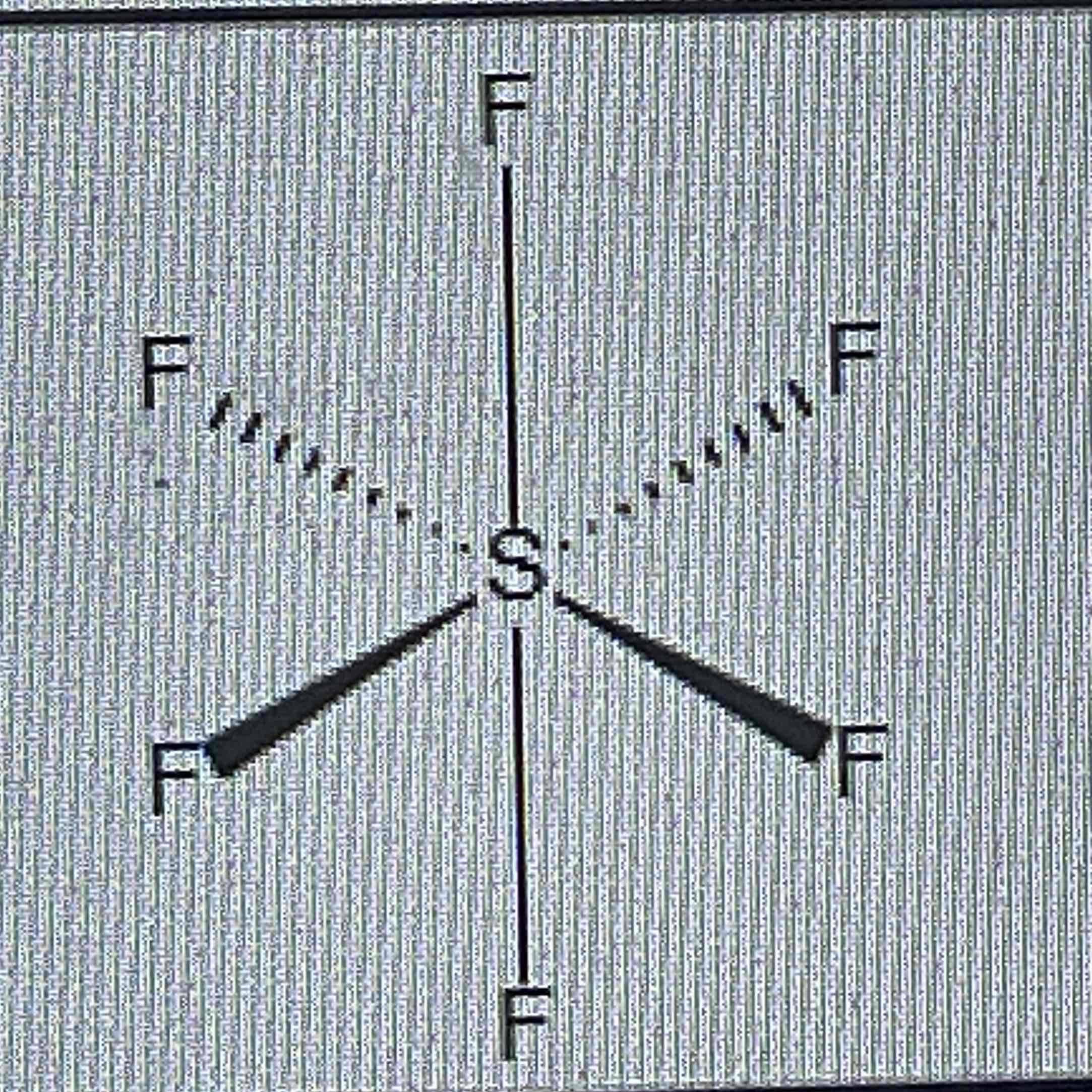
octahedral
No of bond pairs = 6
No of lone pairs = 0
Bond angles = 90º
Example = SF6
Do lone pairs repel more or less than bonding pairs?
More
electronegativity
The relative tendency of an atom in a covalent bond in a molecule to attract electrons in a covalent bond to itself
factors affecting electronegativity
1- increases across a period —> more protons but same no. Of electron shells, hence smaller atomic radius
2- decreases down a group —> distance between nucleus and outer electrons increases and the shielding of inner shell electrons increases
A compound containing elements of similar electronegativity and hence a small electronegativity difference will be
purely covalent
A compound containing elements of very different electronegativity and hence a large electronegativity difference will be
ionic
When is a polar covalent bond formed?
When the elements in the bond have different electronegativities (of around 0.3 to 1.7). When a bond is a polar covalent bond it has an unequal distribution of electrons in the bond and produces a charge separation (dipole).
van der waals forces
A type of intermolecular force that occurs between all molecular substances and noble gases. They do not occur in ionic substances
What is another name for van der waals forces
Transient, induced dipole-dipole interactions
Where do van der waals forces occur
Between all simple covalent molecules and the separate atoms in noble gases
How do van der waals forces work
In any molecule the electrons are moving constantly and randomly. As this happens the electron density can fluctuate and parts of the molecule become more or less negative ie. small temporary or transient dipoles form. These instantaneous dipoles can cause dipoles to form in neighboring molecules. These are called induced dipoles the induced dipole is always the opposite sign of the original one.
What is the main factor affecting van der waals forces?
1- more electrons = higher chance that temporary dipoles will form = van der waals forces stronger between molecules = greater boiling points
Why does the boiling point increase as you go down group 7?
Increasing no of electrons in the bigger molecules causes an increase i the size of the van der waals forces between molecules.
Why does the alkane homologous series have increasing boiling points?
1- more electrons in bigger molecules = stronger van der waals forces
2- long chain alkanes have a larger surface area of contact between molecules for van der waals forces to form compared to spherical shaped branched akanes and so have stronger van der waals forces.
Describe permanent dipole-dipole forces
occur between polar molecules
It is stronger than van der waals forces and so the compounds have higher boiling points
Polar molecules have a permanent dipole
Polar molecules are asymmetrical and have a bond where there is a significant difference in electronegativity between the atoms.
Permanent dipole -dipole forces occur in addition to …
… van der waal forces
Describe hydrogen bonding
Occurs in compounds that ave a hydrogen atom attached to one of the three most electronegative atoms of nitrogen, oxygen and fluorine, which must have an available lone pair of electrons.
Hydrogen bonding occurs in addition to …
… van der waals forces
What is the strongest intermolecular force?
Hydrogen bonding.