Lecture 25 - Cancer Stem Cells and the Tumor Microenvironment
1/61
Earn XP
Description and Tags
ONCOL 335 - Radiobiology. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
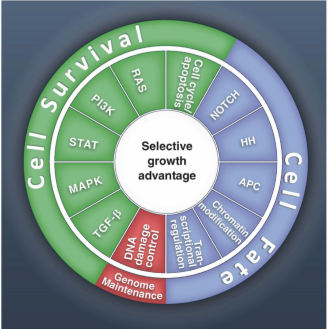
the amount of mutations that tumor has depends on ___
the type of cancer
which cancers have the lowest amount of mutations required
pediatric cancers
which cancers have the most amount of mutations required
adult solid tumors, such as melanoma and lung cancer.
what are the three general areas of pathways the mutations occur in
cell survival
genome maintenance
cell fate
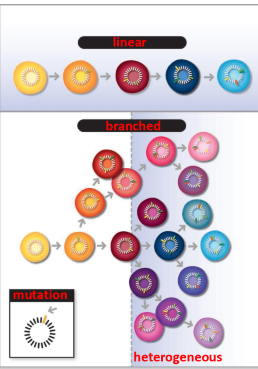
is tumor evolution linear?
No, it is not linear; tumor evolution is often a complex and dynamic process influenced by genetic and environmental factors.
what are initiating mutations
Mutations that first drive the transformation of normal cells into cancerous cells, initiating the tumorigenesis process.
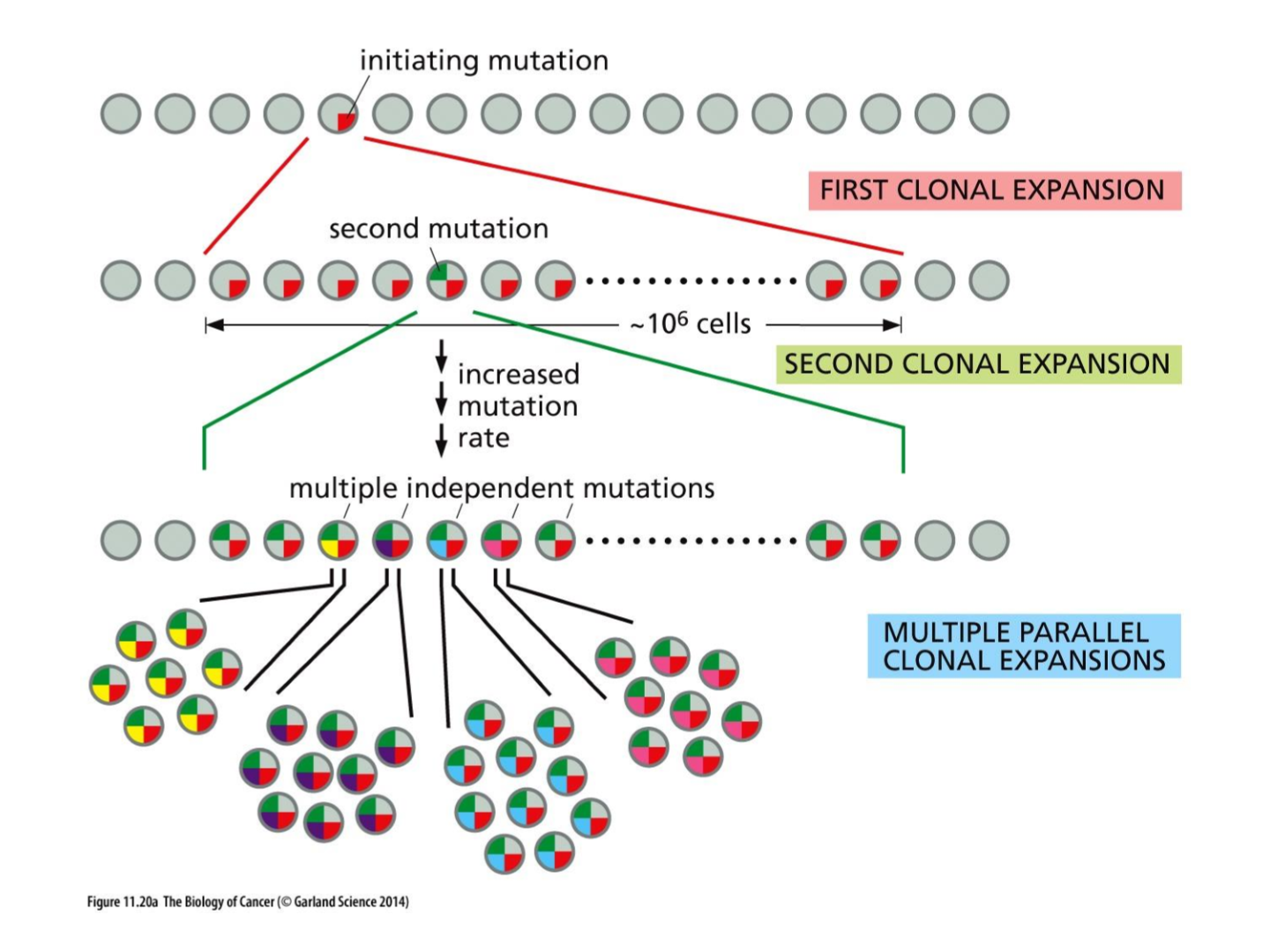
what three things effect cancer evolution
mutations
selection
niches
what is intratumoral heterogeneity
The presence of distinct subpopulations of cells within a tumor, each with different genetic, phenotypic, or functional characteristics, contributing to tumor complexity and treatment resistance.
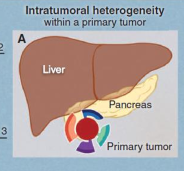
what is intermetastatic heterogeneity
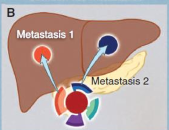
what is intrametastatic heterogeneity
The variation in cellular characteristics observed within individual metastatic tumors, reflecting differences in genetic and phenotypic traits that arise during the metastatic process.
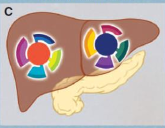
what is interpatient heterogeneity
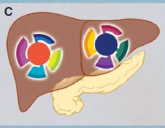
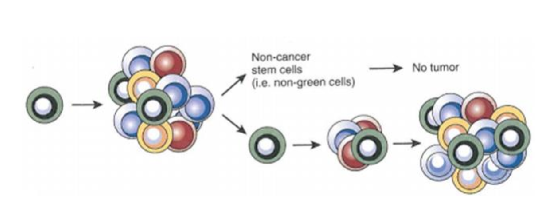
list two models of tumor propagation
classical stochastic model
cancer stem cell model
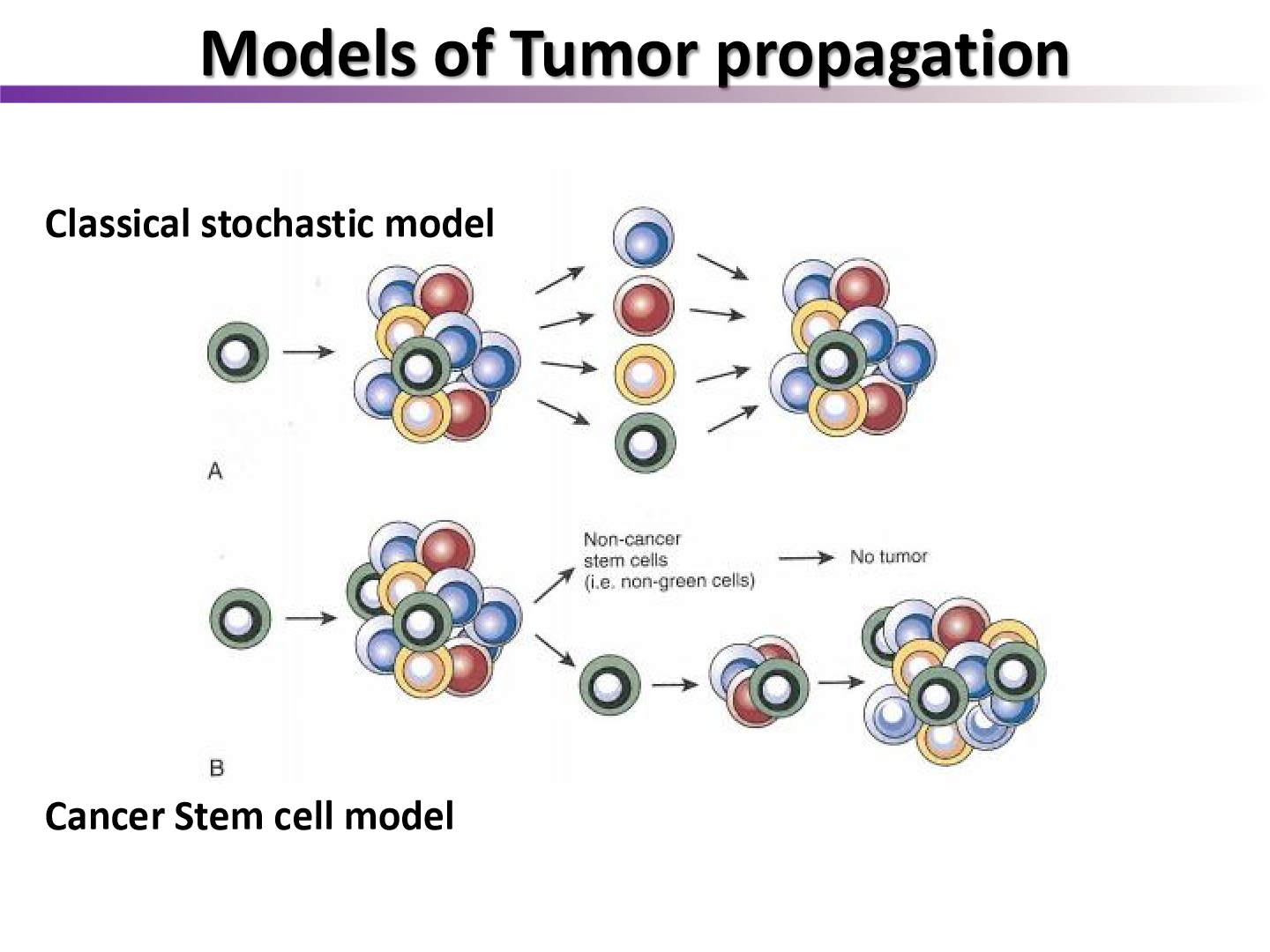
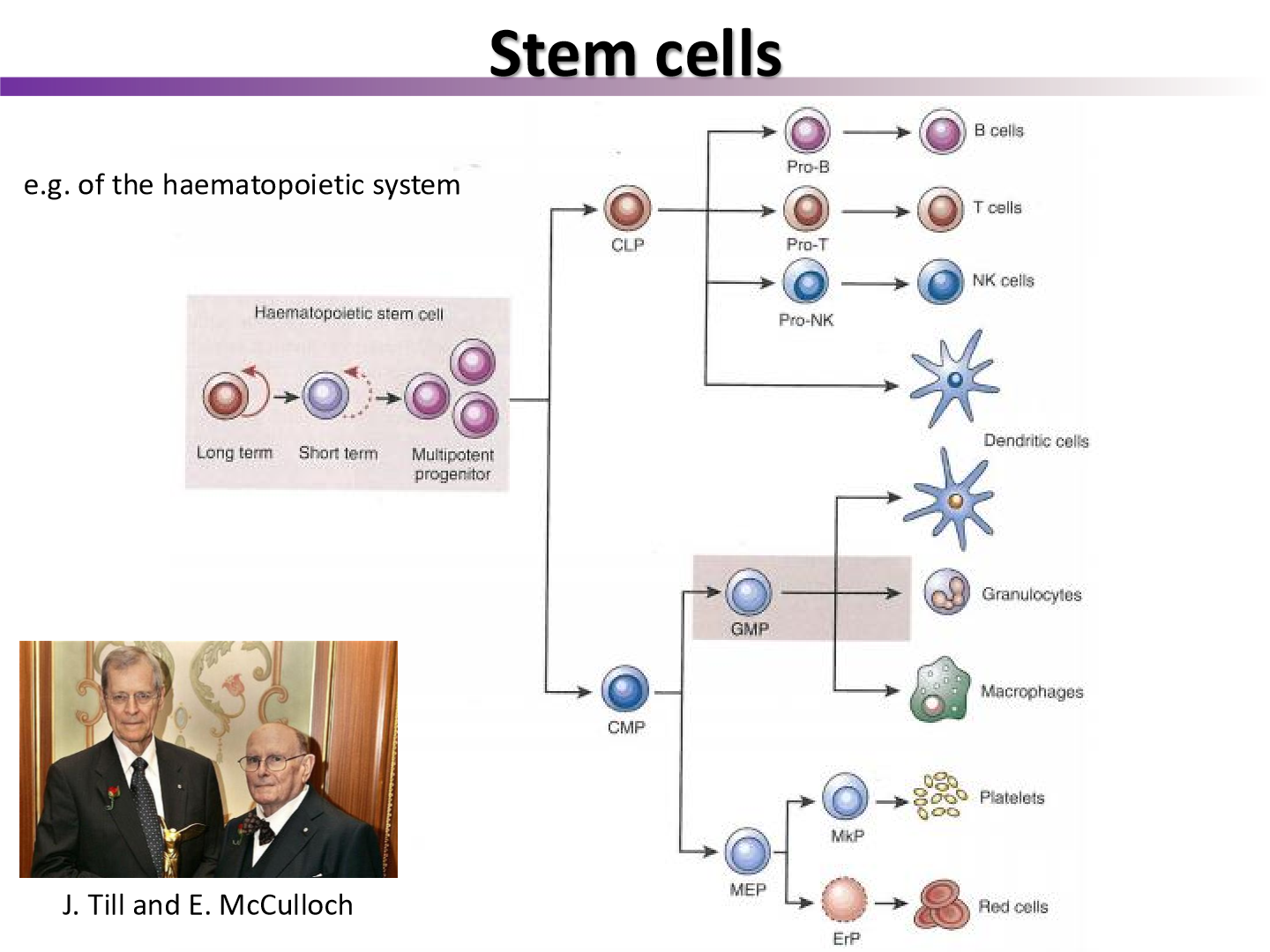
what experiment was the cancer stem cell model derived from
the Till and McCulloch experiment
what did Rudolf Virchow theorize regarding the origin of cancer
undifferentiated cells may be the origin of cancers
what percent of tumors do CSC (tumor initiating cells) make up
1-5%
what experiment can be done to prove that it requires ~100-fold fewer TICs to generate a tumor than bulk cells
limiting dilution assay
if a chemo drug kills off all the cells except the TIC, what occurs
tumor can grow back
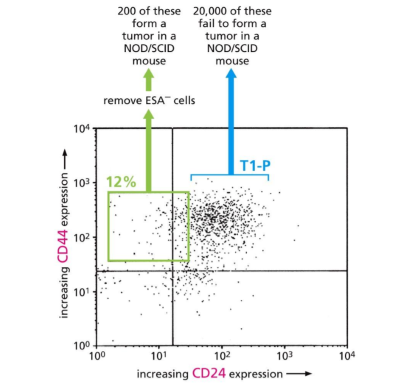
what does this graph tell us
High CD44 and low CD24 gets tumor
But high CD44 and CD24 get low tumor
Therefore CD44 is the CSC
describe a TLD50 assay
A TLD50 assay measures the dose of a drug that is lethal to 50% of a test population, commonly used to evaluate the potency of cytotoxic agents in cancer research.
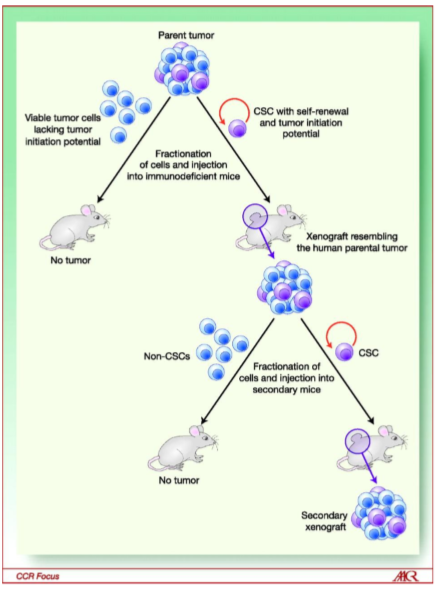
what are tumorspheres
Tumorspheres are clusters of cancer cells that grow in a three-dimensional culture, reflecting the characteristics of in vivo tumors, and are often used to study cancer stem cells and their properties.
name of CSC in H+N cancer
CD44
name of CSC in glio and medulloblastomas
CD133
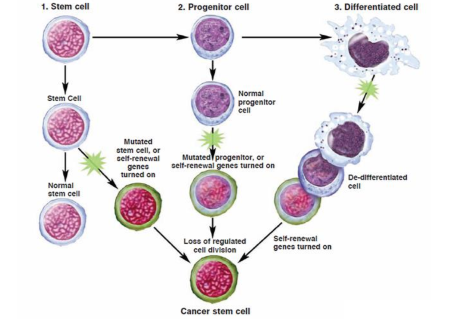
Do cancer stem cells after to originate from normal stem cells
no, we are not sure where they come from.
can come from stem cells, progenitor cells, or differentiated cells
What is the tumor microenvironment
The tumor microenvironment refers to the surrounding cells, signaling molecules, and blood vessels that interact with tumor cells and influence their growth and behavior. It plays a crucial role in tumor progression, metastasis, and treatment response.
surrounding cells influence the tumor cells and their growth, differentiation, and survival.
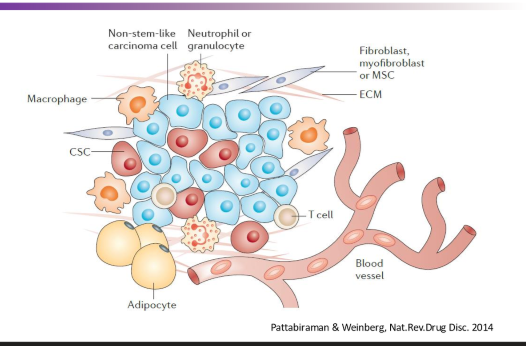
interactions between tumor cells and their environment can be ….
postive or negative
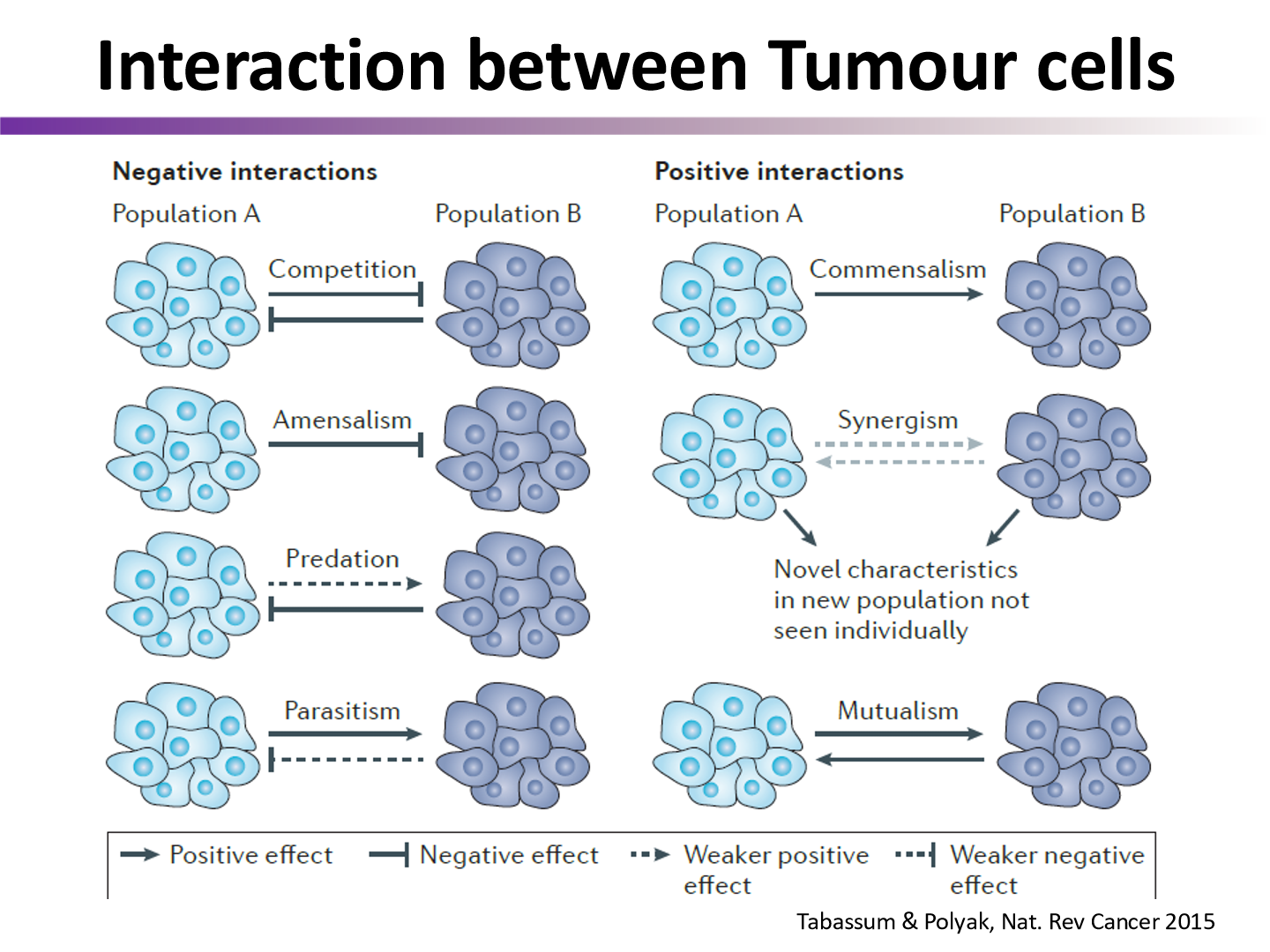
what are the two ways cancer cells can ‘talk’ to eachother
juxtacrine signals
paracrine signals
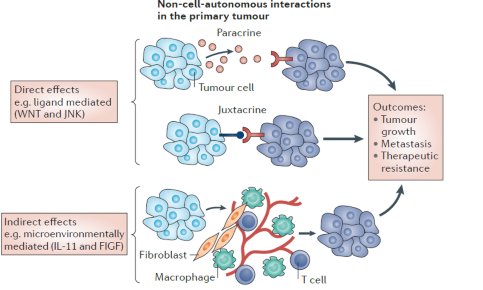
what are the two biggest issues of treating cancers
metastasis
resistance to treatment
if a cancer wants to metastasize, what needs to occur?
it needs ot undergo EMT
What is epithelial-mesenchymal transition (EMT)?
Epithelial-mesenchymal transition (EMT) is a process where epithelial cells lose their cell polarity and adhesion properties and acquire mesenchymal, migratory characteristics. EMT is crucial in embryogenesis, wound healing, and fibrosis, and it plays a role in cancer metastasis by enabling tumor cells to invade surrounding tissues.

what is cell plasticity
the ability of cells to change their phenotype without genetic mutations in response to environmental cues
do stem cells have low or high plasticity
high
in regards to the CSC model, what do we need to target to reduce metastasis and primary tumors>
the tumor initiating cells
prevent relapse and metastasis with CSC therapy
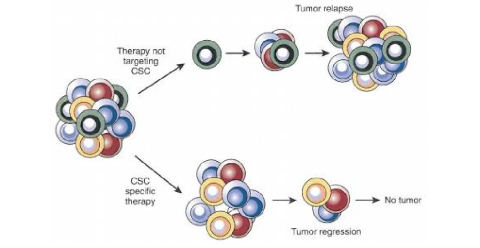
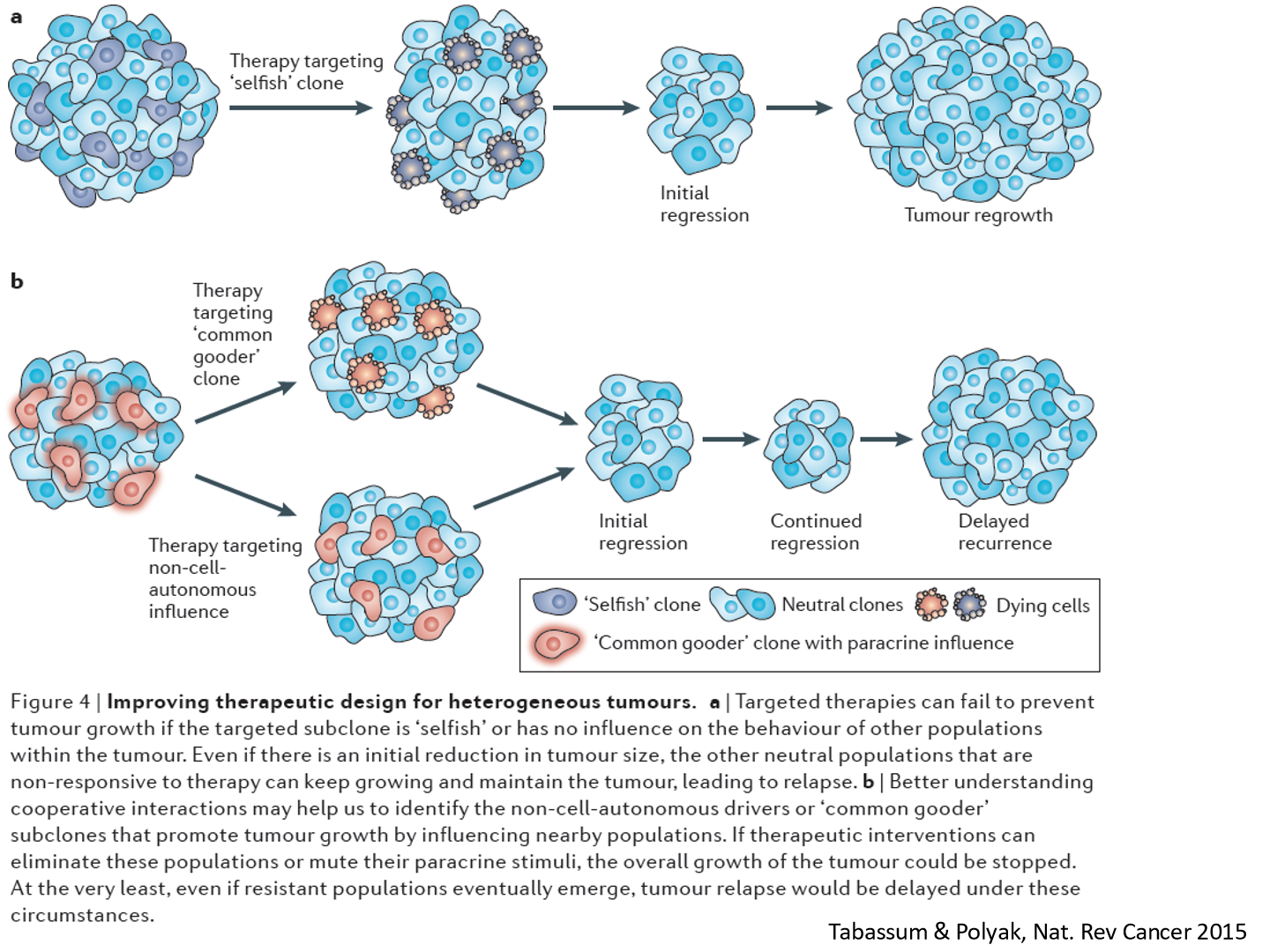
what else can be done to reduce CSCs in a tumor
If we can reduce the interaction between the clones, we can reduce the tumor
clones will signal to eachother and help the tumor, we want to prevent that
target the clones that effect other clones positively
are CSC more radioresistant than bulk tumor cells?
yes
tumors with CD133 present will create more tumors after irradiation
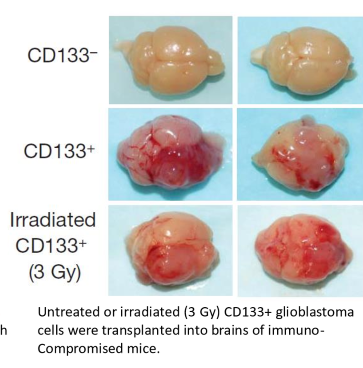
do CSC have faster repair than bulk tumor cells?
yes
ATM and ATR are more stronger activated in CSCs
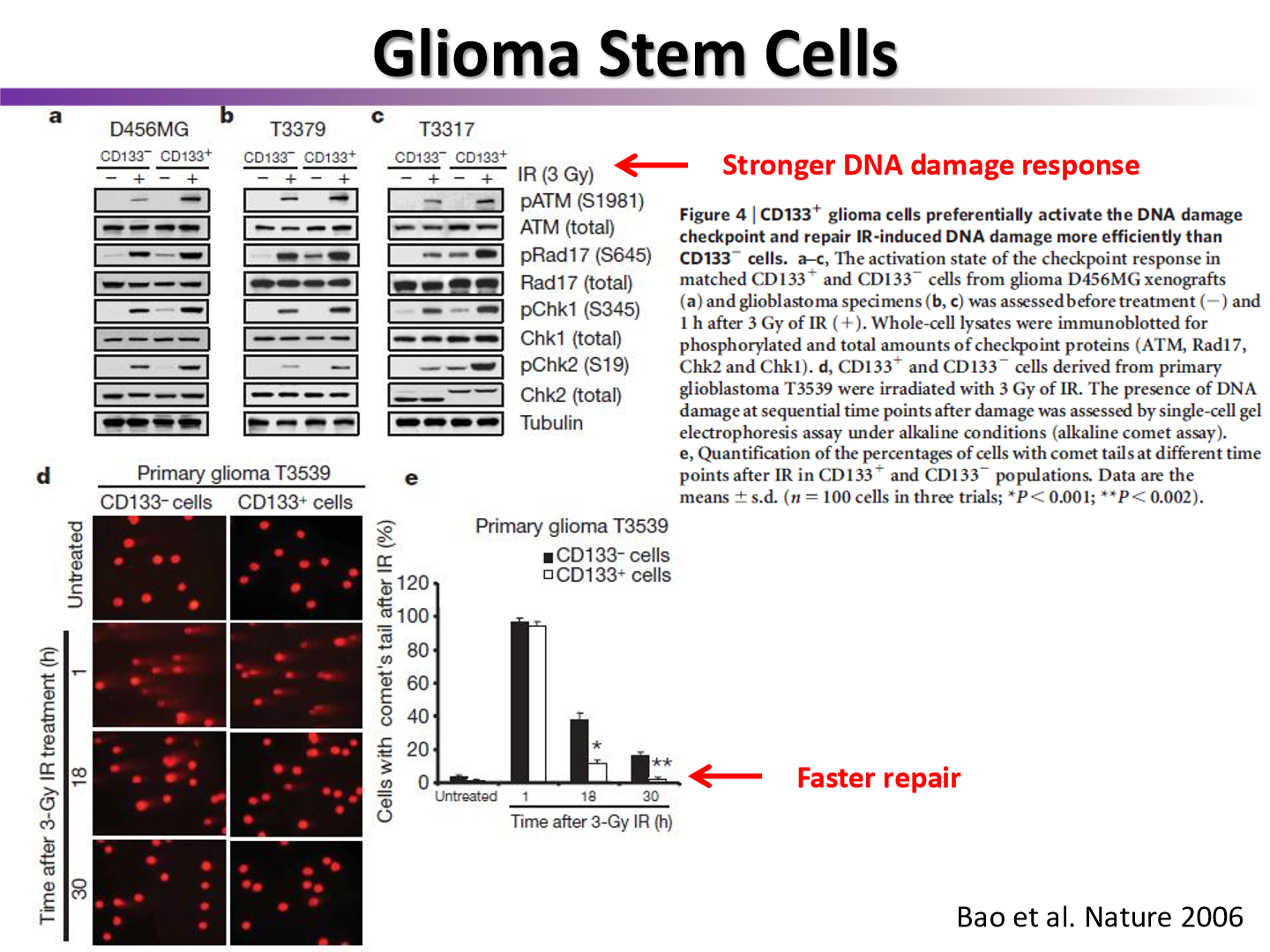
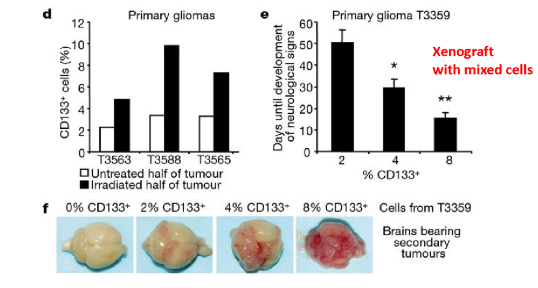
the more CSC …
the more propensity to make a viable tumor
are CSCs more immune to apoptosis
yes
they are less likely to be forced into apoptosis after irradiation
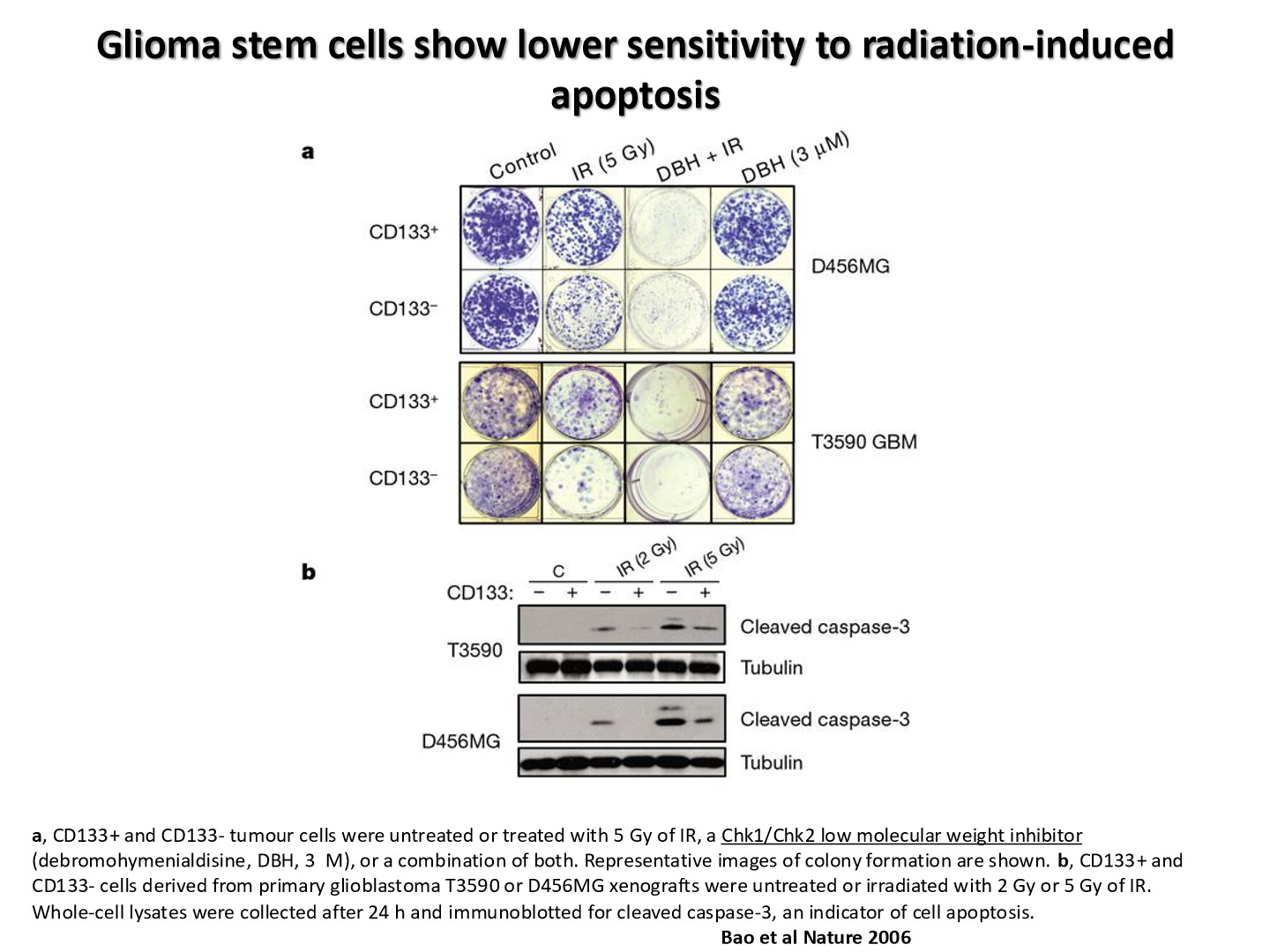
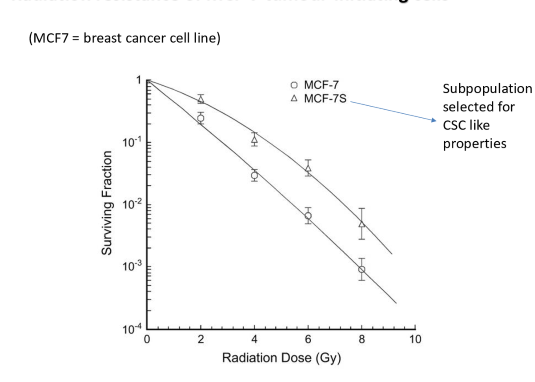
what does this graph tell us
CSC are more radioresistant than normal tumor bulk cells
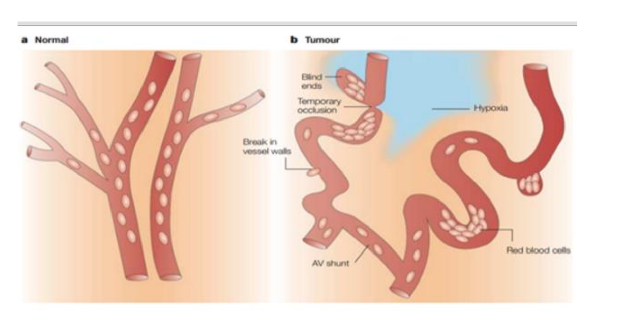
what are the two types of hypoxia that occur in tumors
chronic hypoxia
acute hypoxia
chronic hypoxia
results from a limited diffusion distance of oxygen through respiring tissue
acute hypoxia
result of temporary closure of tumor blood vessel due to malformed vasculature
tumor has leaky blood vessels
what was the 1955 Thomlinson and Gray experiment discover
oxygen can diffuse roughly 70 um
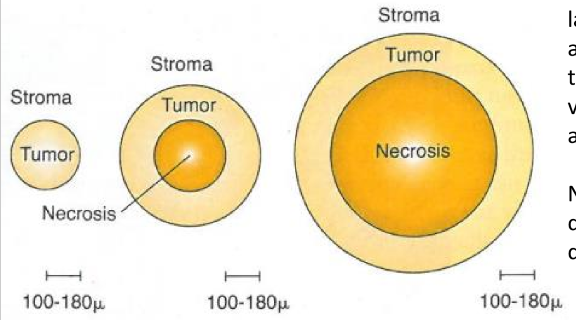
as the tumor cord grows…
the necrotic center also enlarges, so that the thickness of the sheath of viable tumor cells remains approximately constant
what has better diffuusion, arterioles or venuoles
arterioles
more oxygen present
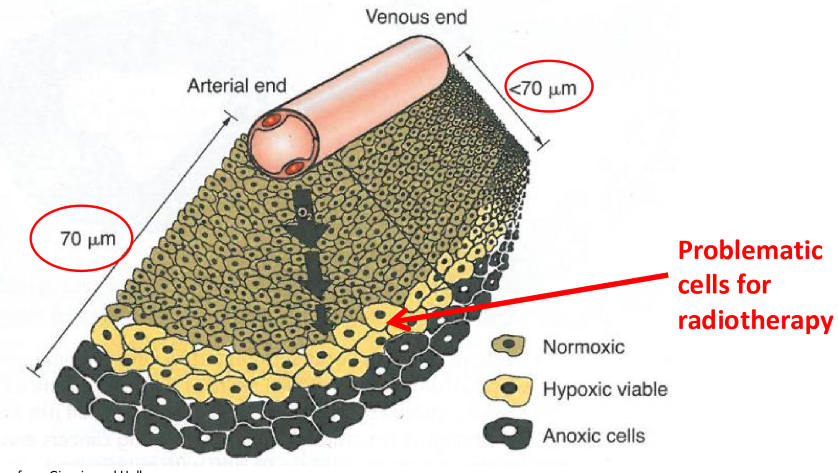
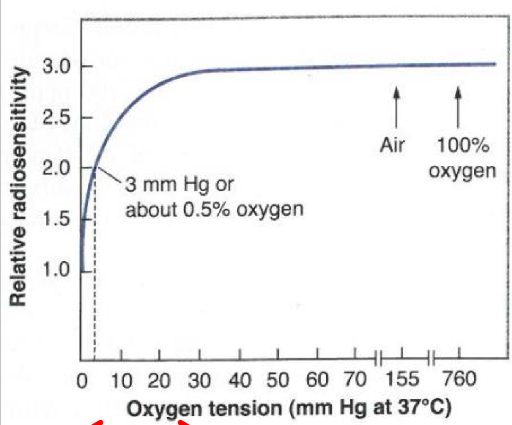
what does this graph tell us
by the time an O2 concentration has reached roughly 5%, the survival curve is virtually indistinguishable from that under normoxia
increasing O2 from 5%-100% has little affect
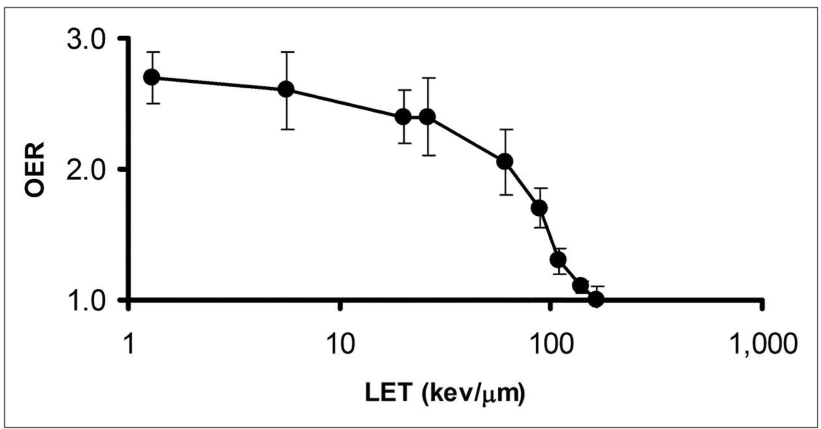
why does OER have no effect on high LET radiation
Because high LET radiation causes damage regardless of oxygen presence, meaning that the oxygen enhancement ratio is not applicable.
hypoxia is sensed by what transcription factor?
HIF-1a
function of transcription factor
- Protein that recruits transcription machinery to DNA
- Regulates activation of the genes
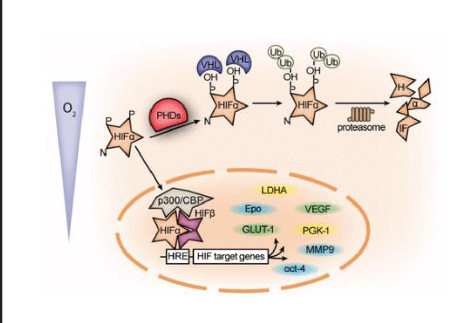
describe what happens when hypoxia is detected
in prescence of oxygen, HIF1a is hydroxylated by VHLs causing Ubiquitiation and degradation (works same as MDM2 and p53)
without oxygen, HIF-1a is not degradedand translocates to the nucleus, where it dimerizes with HIF-1β, and binds to HRE
leads to gene transcriptition
what genes does HIF1a activated
VEGF (for blood vessel growth)
apoptosis
EPO
Leptin
what effect does HIF-1a have on the EMT
HIF-1a promotes the epithelial-to-mesenchymal transition (EMT) by activating genes that enhance cell migration and invasion, contributing to metastasis in cancer.
hypoxia is one of the driving forces to faciliate EMT
if stem cells are more plastic than bulk tumor cells, what can be said about their levels of HiF-1a
they have higher levels of HIF-1a, resutling in increase EMT
what happens to stem cells if you inhibit HIF-1a with ShRNA?
Inhibiting HIF-1a with ShRNA leads to reduced stem cell properties, decreased EMT, and diminished tumorigenic potential.
less neurospheres formed in vitro
less tumors in vivo
3 reasons why hypoxia is bad for radiation therapy
oxygen effect
HIF-1a plays role in cancer stem cell renewal
HIF-1a enhances EMT
list 6 signalling pathways that are used by cancer stem cells and may be theoretically inhibited by cancer drugs
WNT
TGF-B
Notch
Hedgehog
JAK-STAT
PDGFR
what signalling pathways theoretically could Salinomycin target
WNT and maybe mTOR/AKT
has any drug made to target cancer signalling pathways made it to clinic?
no, they have not made it past stage 1 trials
all of the drugs tried are used to treat other issues, like malaria or bacteria
what is the reason as to why a drug targeted cancer stem cells is not working for treatment
a bulk cancer cell may have the potential to change it’s phenotype and become a stem cell in the abscence of other stem cells
this has been proven scientifically: non-CSC have been able to form tumors
what may be another way to target cancer stem cells
by inhibiting their DNA damage response mechanisms
since they are good at this
why will targeted DNA damage response of tumor cells not negatively impact our healthy cells
since cancer has DNA repair defects in pathways, they often rely heavily on the intact repair pathways, whereas normal cells can use other pathways
ex: cancers use ATR repair more than normal cells, so if a drug is created to inhibit ATR, the cancer cell may die
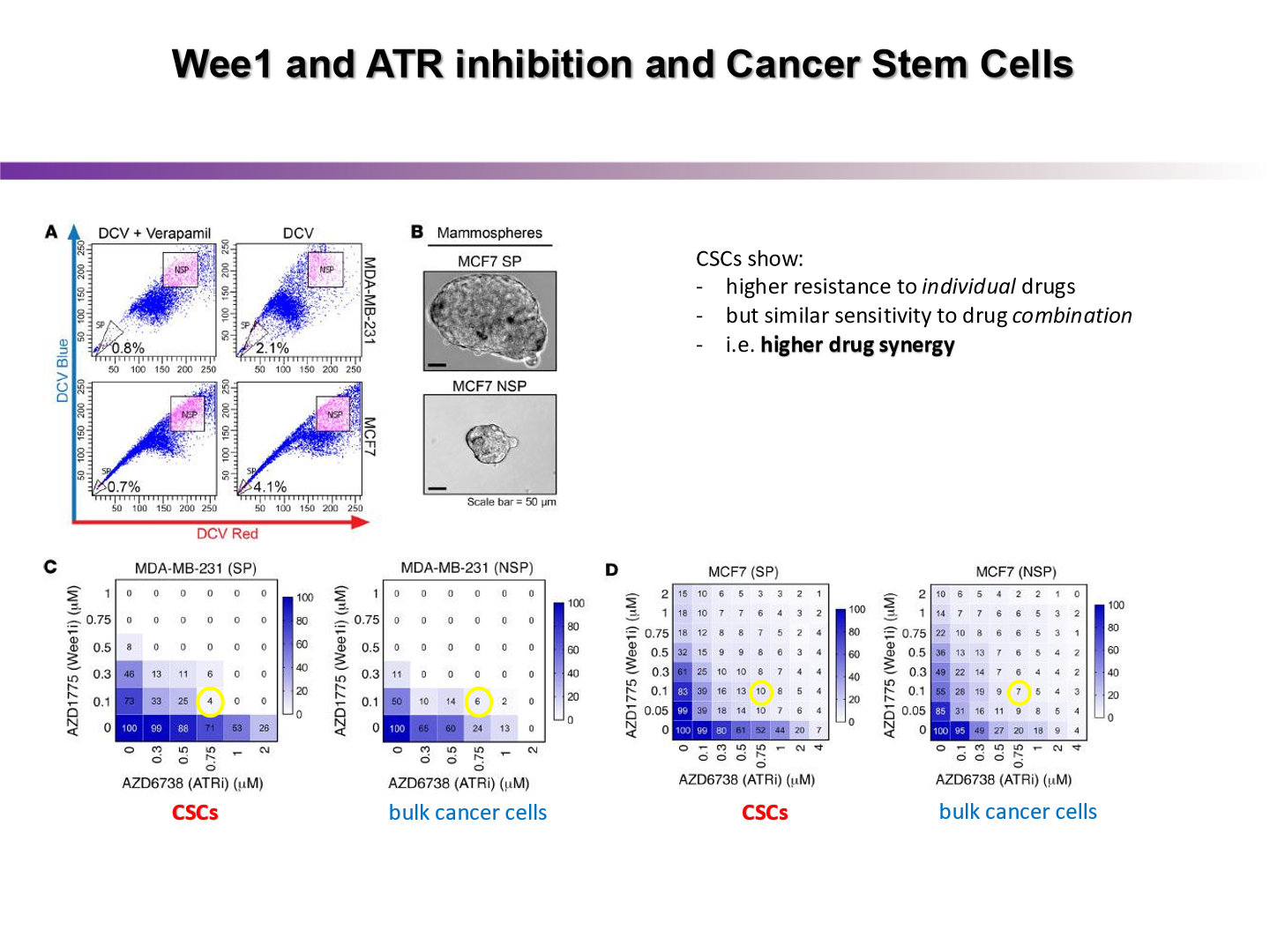
what did this experiment show us
by pairing a drug that inhibits ATR with a drug that forces the cancer cell into mitosis, the cancer cell kill worked quite well
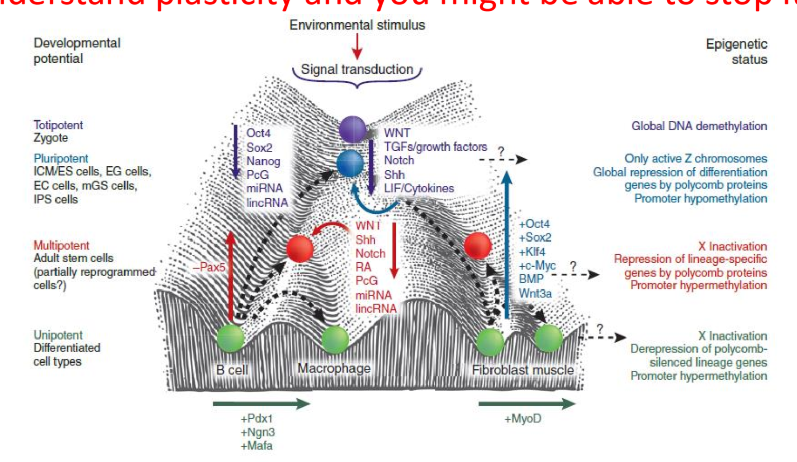
what do we need to understand before being able to target cancer stem cells
we need to understand plasticity and epigenetics
cells can change their phenotype to change into CSCs, so if we understand plasticity, we may know how to target it better