Chemistry II Module of the MCAT Self Prep eCourse: Lesson 2: VSEPR Part II and Chirality (Pro)
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Lesson 2: VSEPR Part II and Chirality
Lesson 2: VSEPR Part II and Chirality
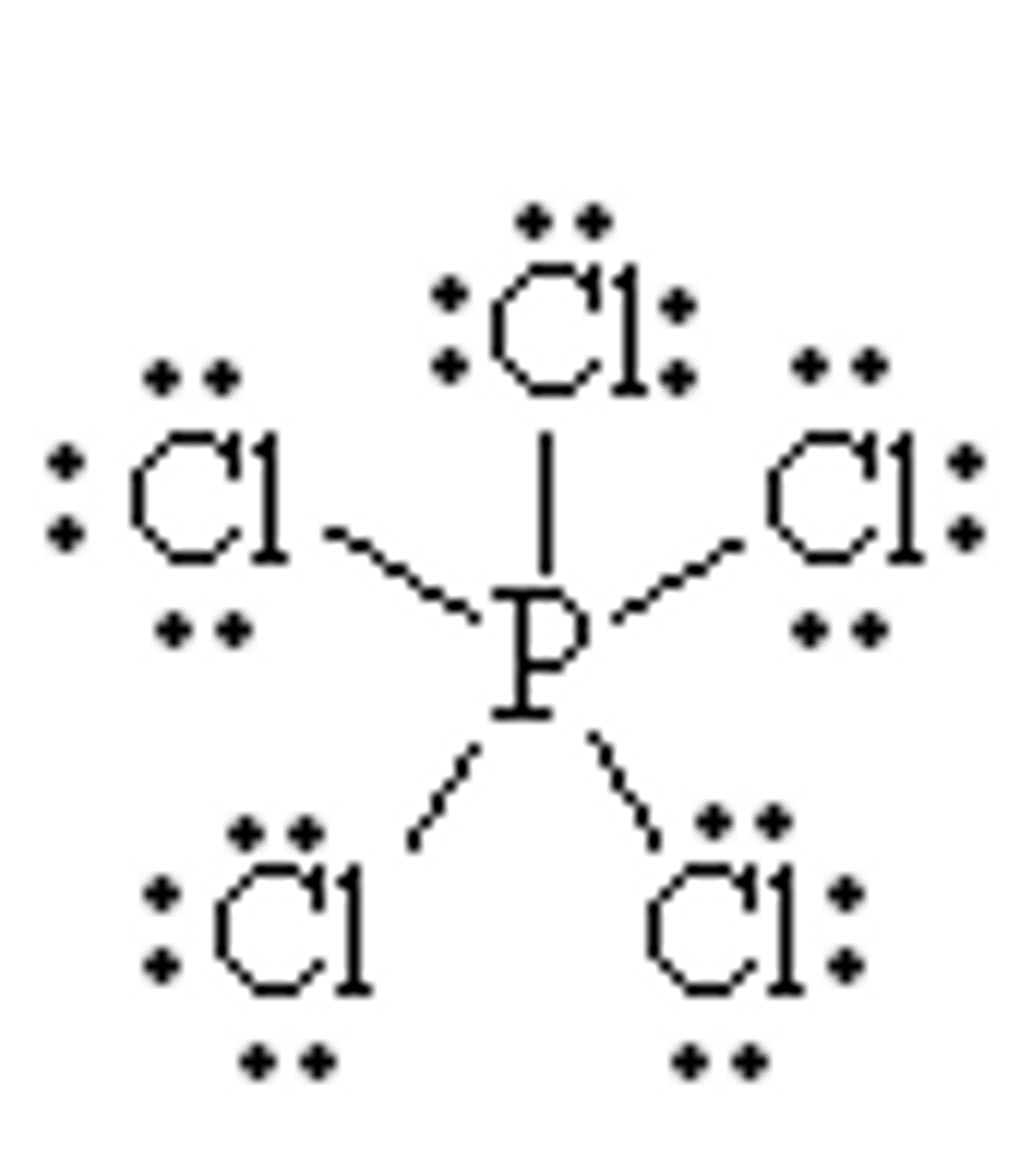
What is the Electron Geometry of PCl5? Molecular Geometry?
Periodic table for reference: http://www.chem.qmul.ac.uk/iupac/AtWt/table.gif
Electron Geometry of PCl5 is Trigonal Bipyramidal.
Molecular Geometry of PCl5 is Trigonal Bipyramidal.
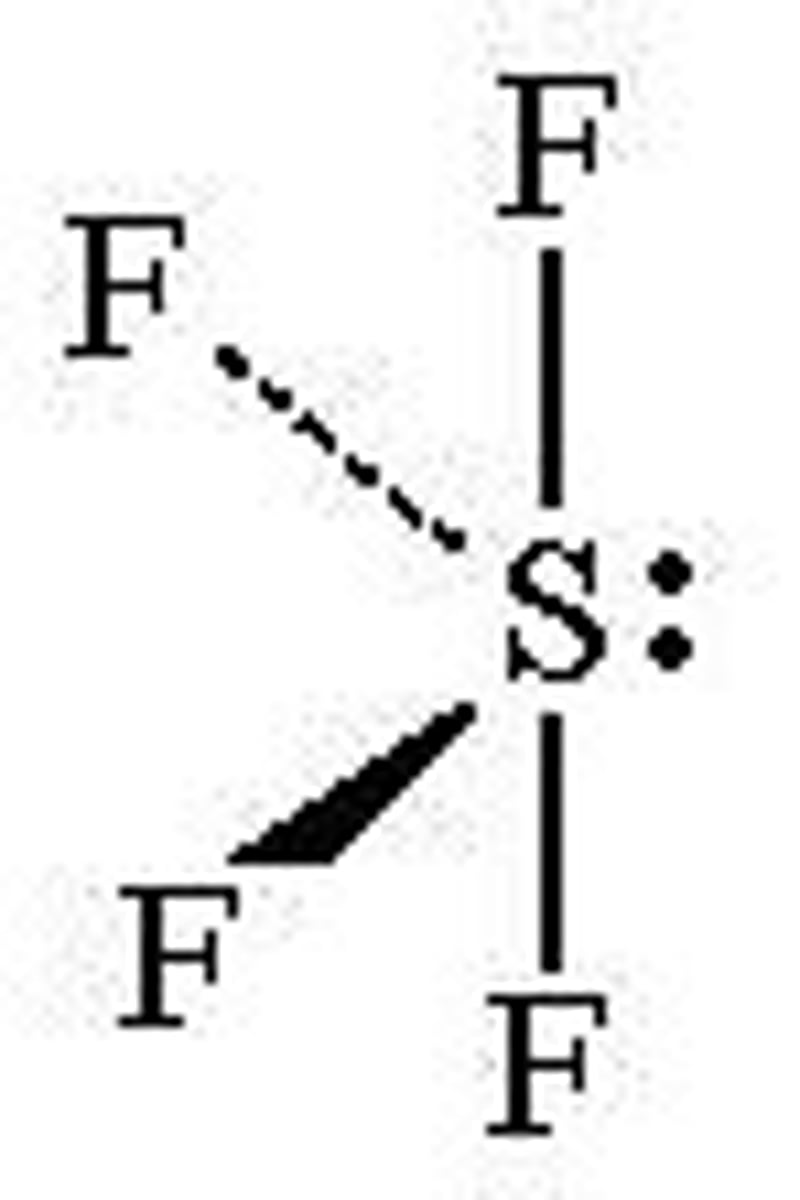
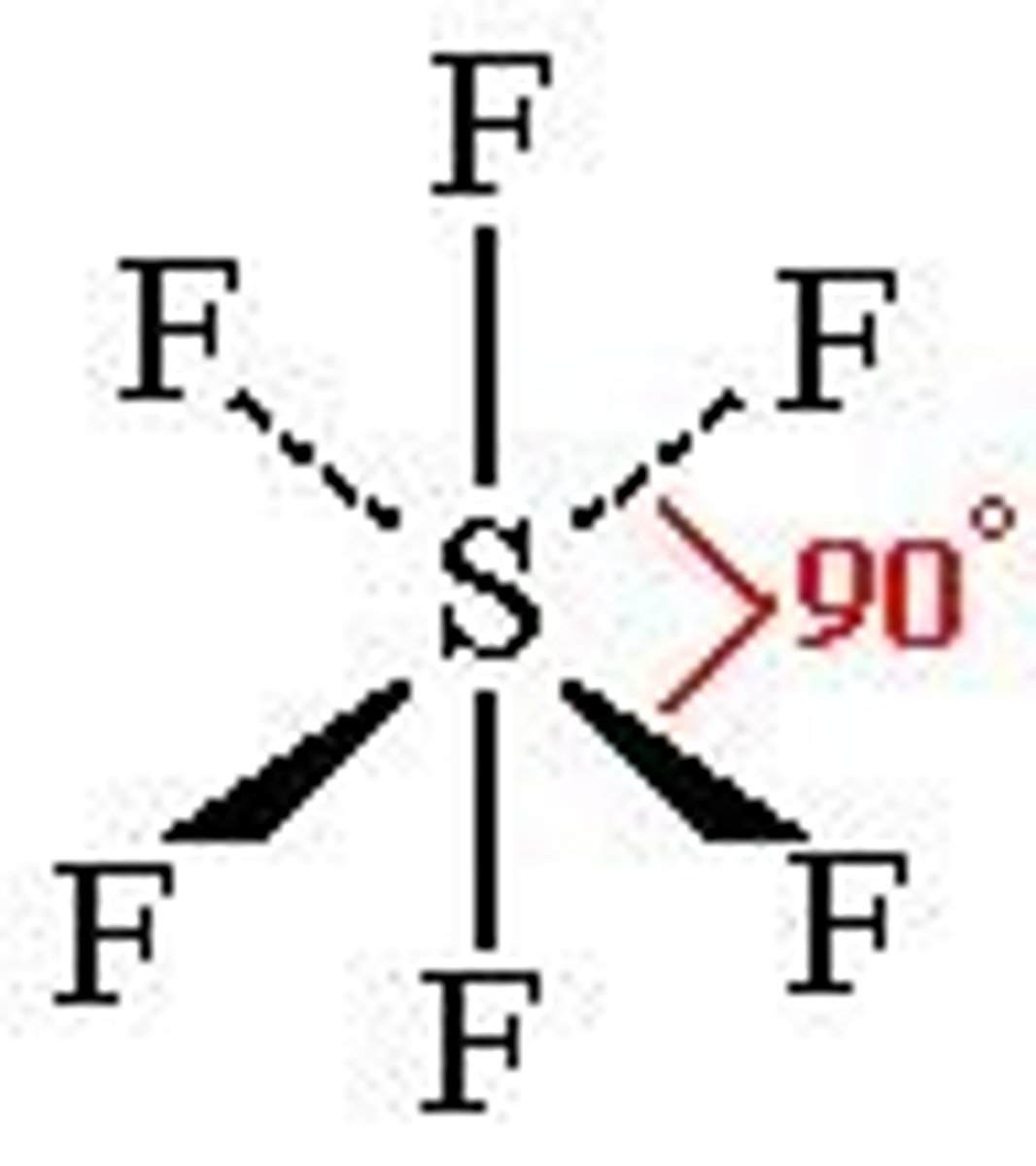
What is the Electron Geometry of SF4? Molecular Geometry?
Electron Geometry of SF4 is Trigonal Bipyramidal.
Molecular Geometry of SF4 is See-saw.
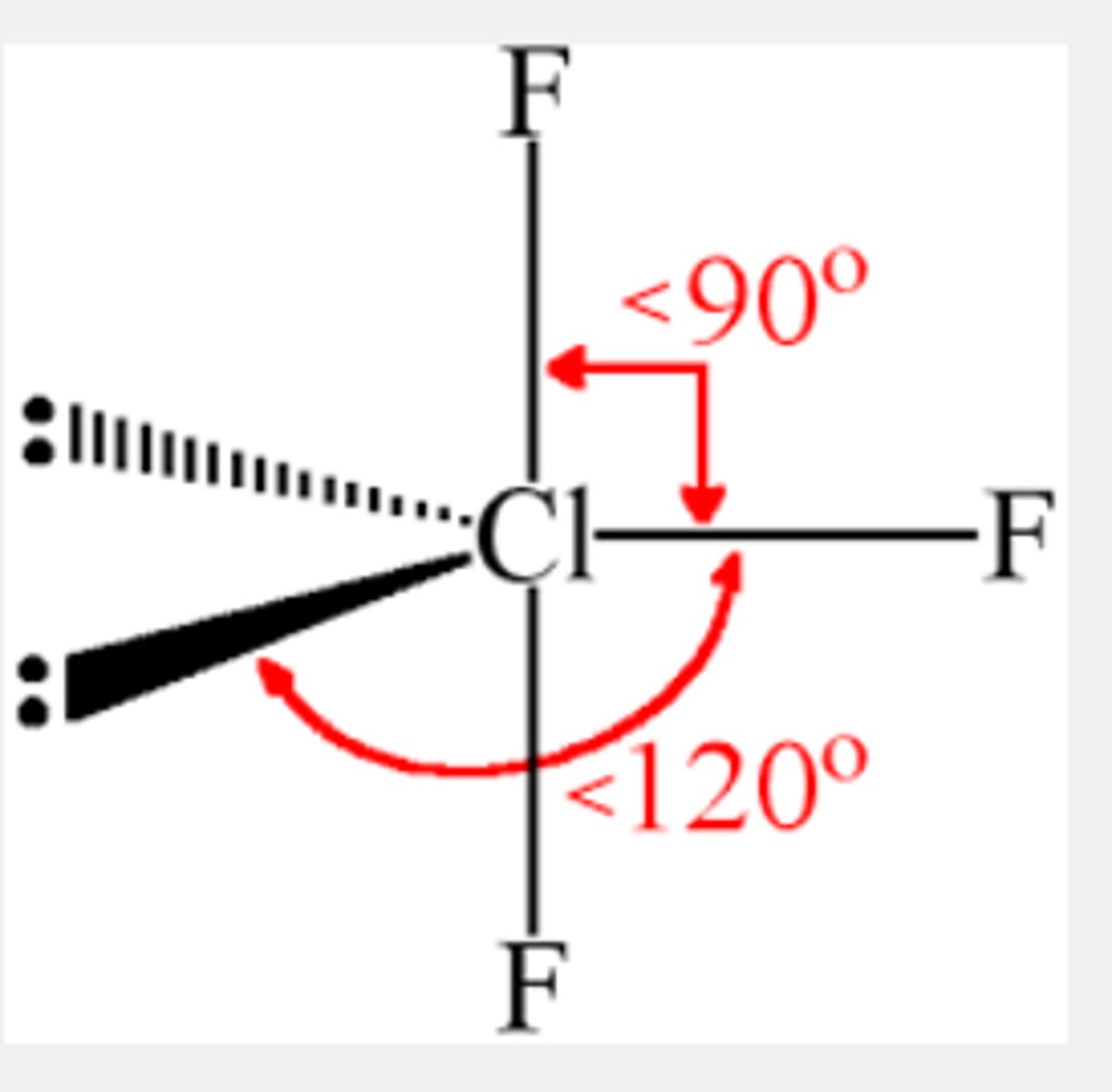
What is the Electron Geometry of ClF3? Molecular Geometry?
Electron Geometry of ClF3 is Trigonal Bipyramidal.
Molecular Geometry of ClF3 is T-shaped.
What is the Electron Geometry of I3-? Molecular Geometry?
Electron Geometry of I3- is Trigonal Bipyramidal.
Molecular Geometry of I3- is Linear.
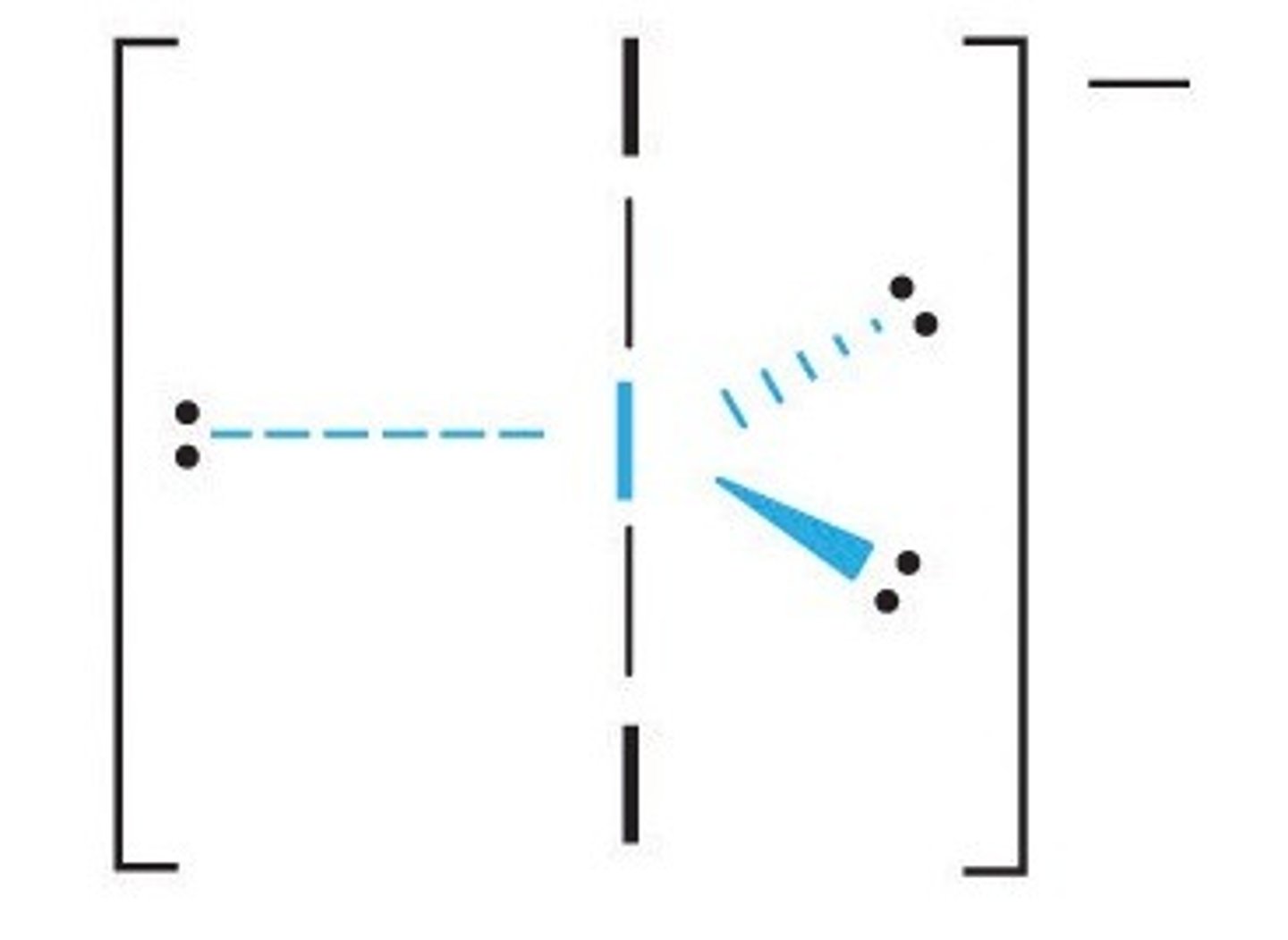
What is the Electron Geometry of SF6? Molecular Geometry?
Electron Geometry of SF6 is Octahedral.
Molecular Geometry of SF6 is Octahedral.
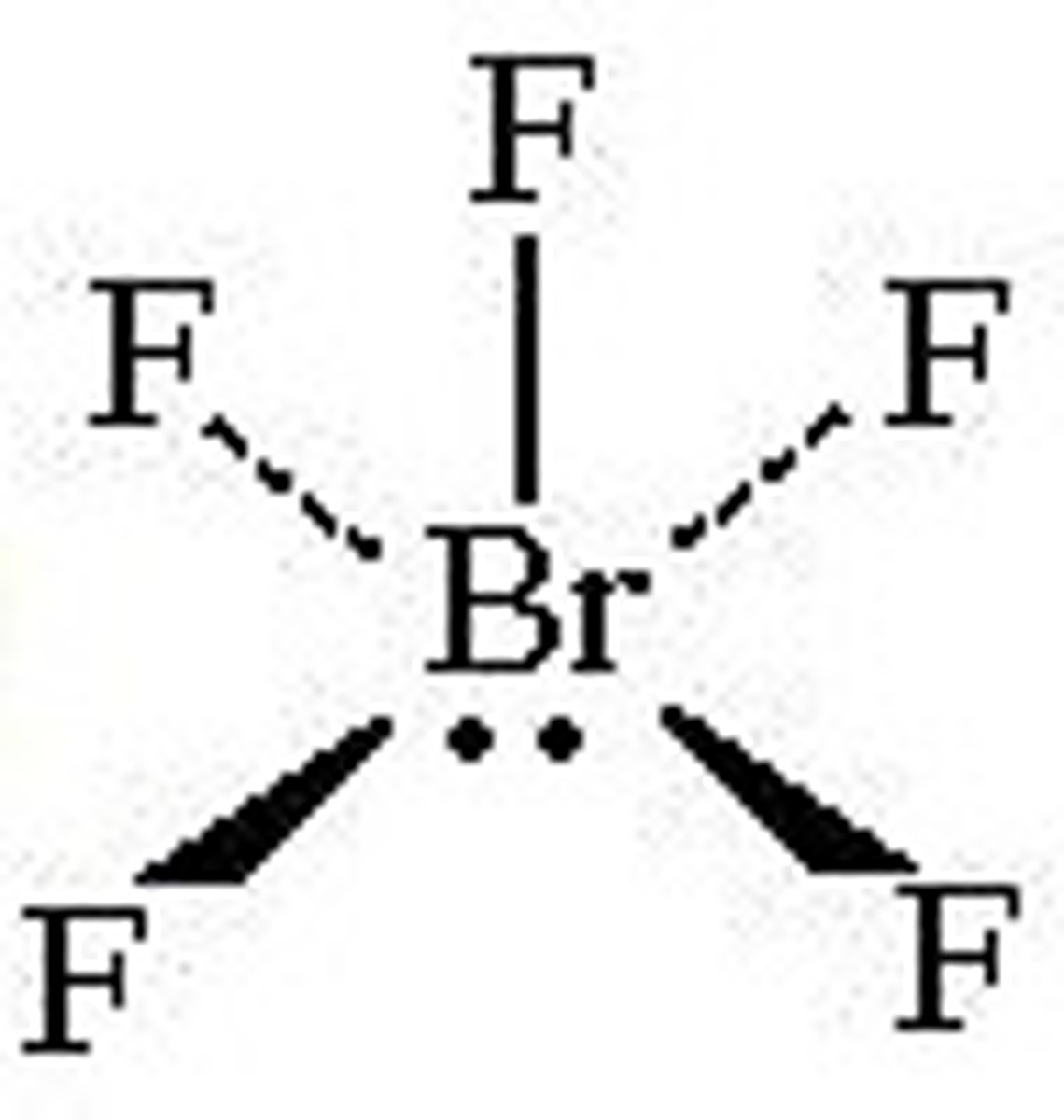
What is the Electron Geometry of BrF5? Molecular Geometry?
Electron Geometry of BrF5 is Octahedral.
Molecular Geometry of BrF5 is Square Pyramidal.
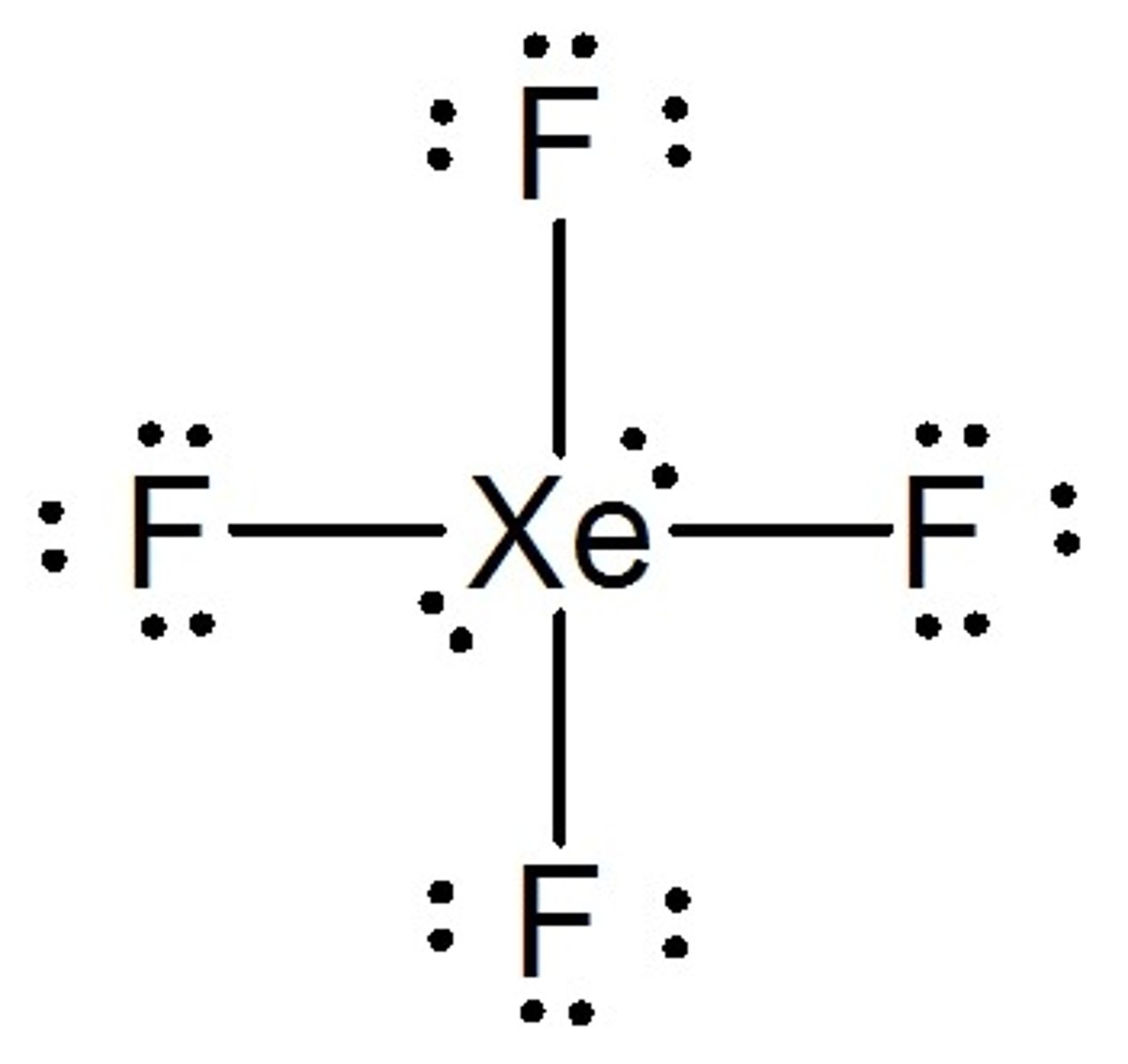
What is the Electron Geometry of XeF4? Molecular Geometry?
Electron Geometry of XeF4 is Octahedral.
Molecular Geometry of XeF4 is Square Planar.
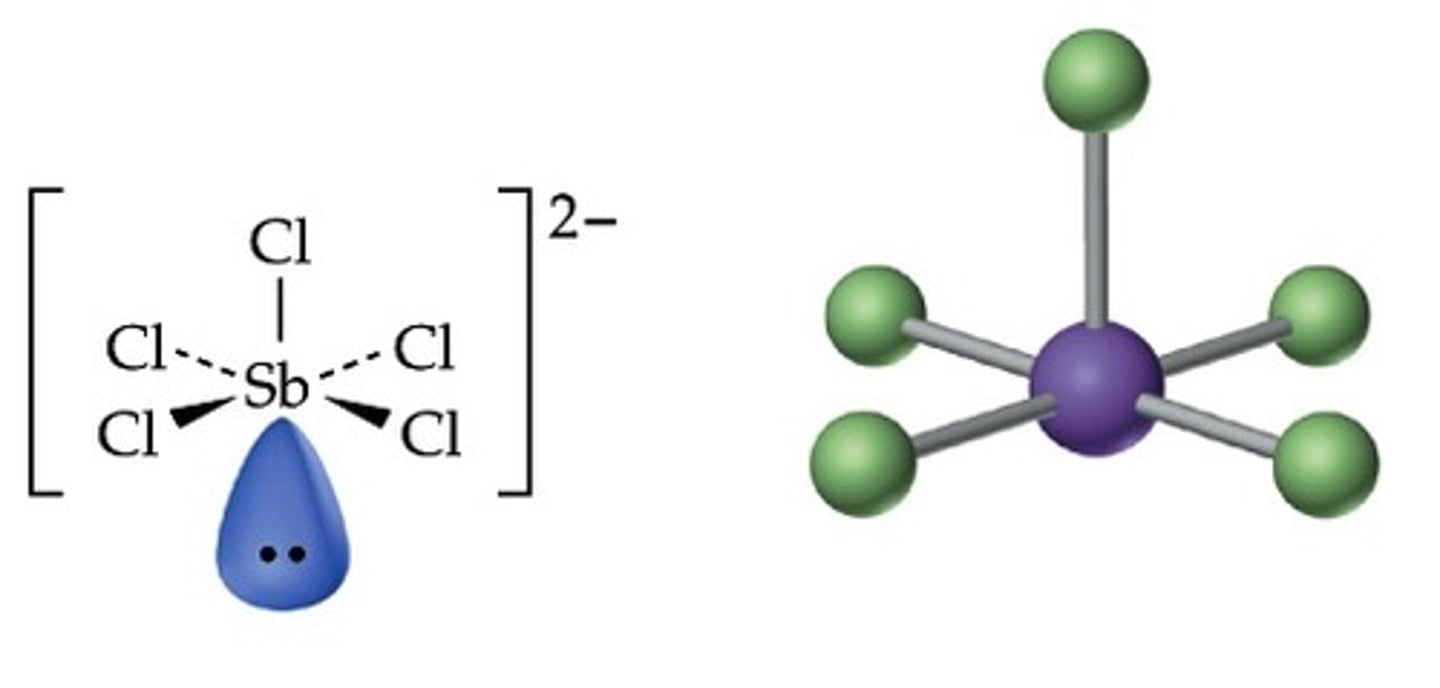
What is the Electron Geometry of SbCl5(2-)? Molecular Geometry?
Electron Geometry of SbCl5(2-) is Octahedral.
Molecular Geometry of SbCl5(2-) is Square Pyramidal.
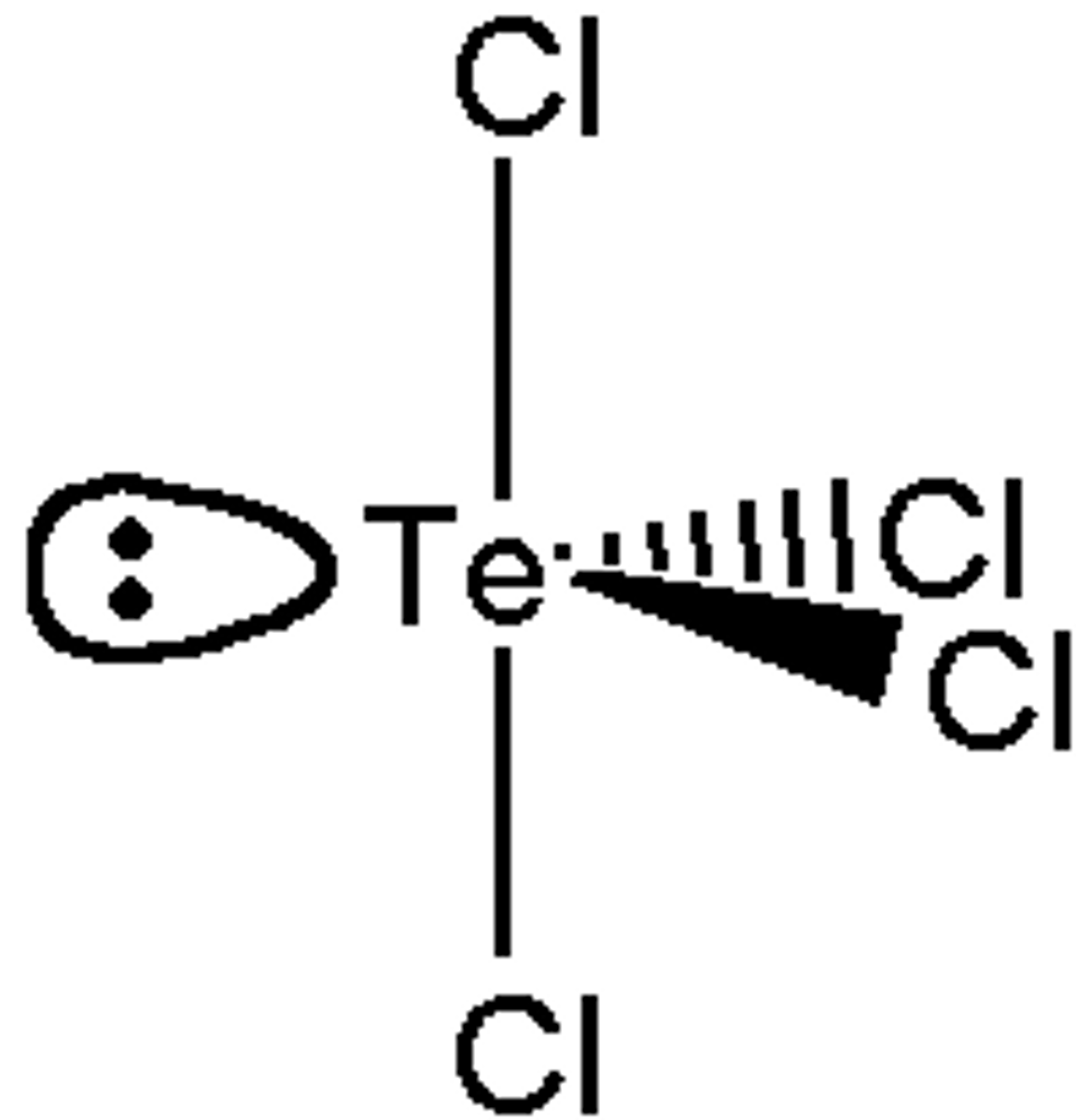
What is the Electron Geometry of TeCl4? Molecular Geometry?
Electron Geometry of TeCl4 is Trigonal Bipyramidal.
Molecular Geometry of TeCl4 is See-saw.
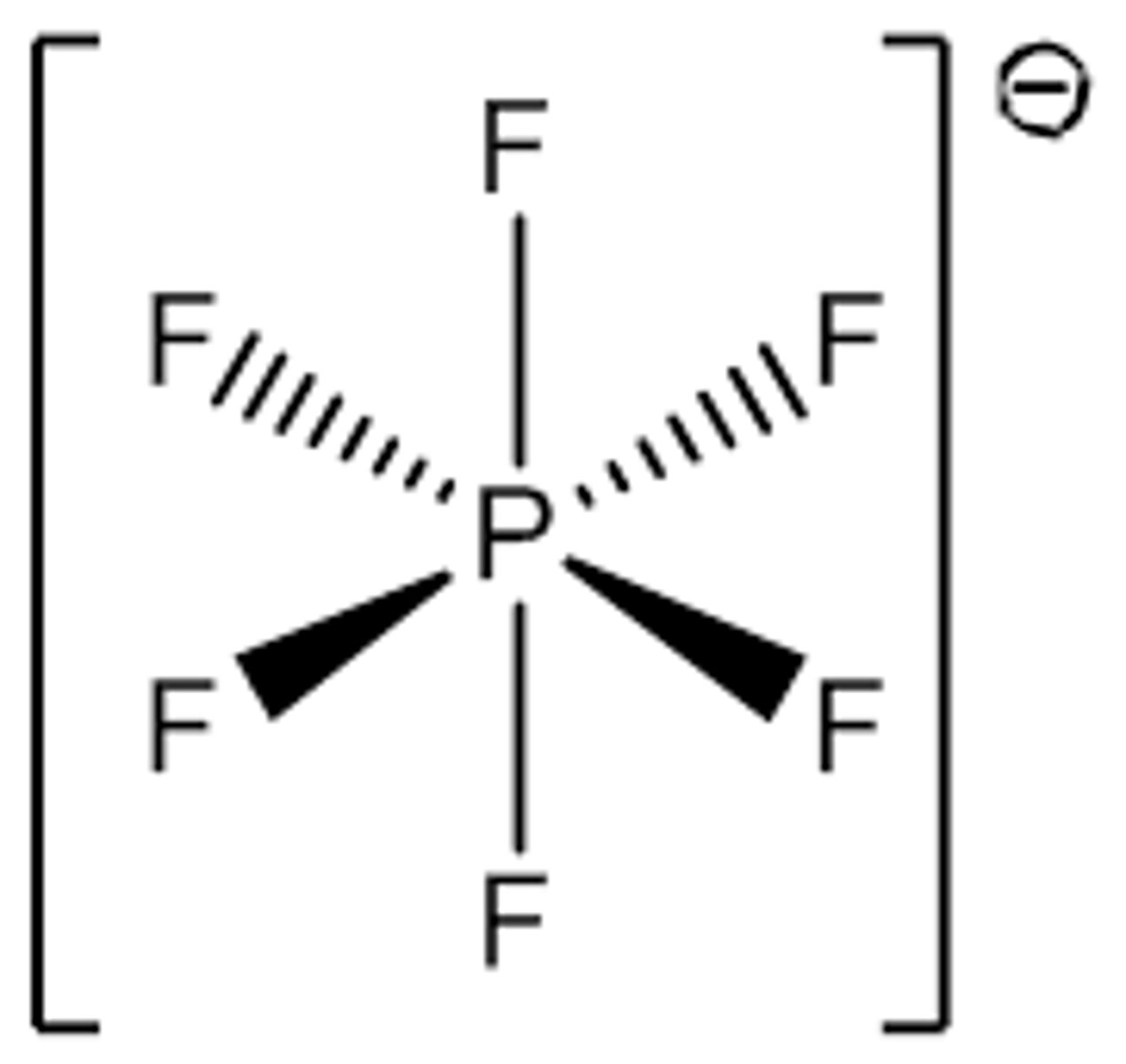
What is the Electron Geometry of PF6-? Molecular Geometry?
Electron Geometry of PF6- is Octahedral.
Molecular Geometry of PF6- is Octahedral.
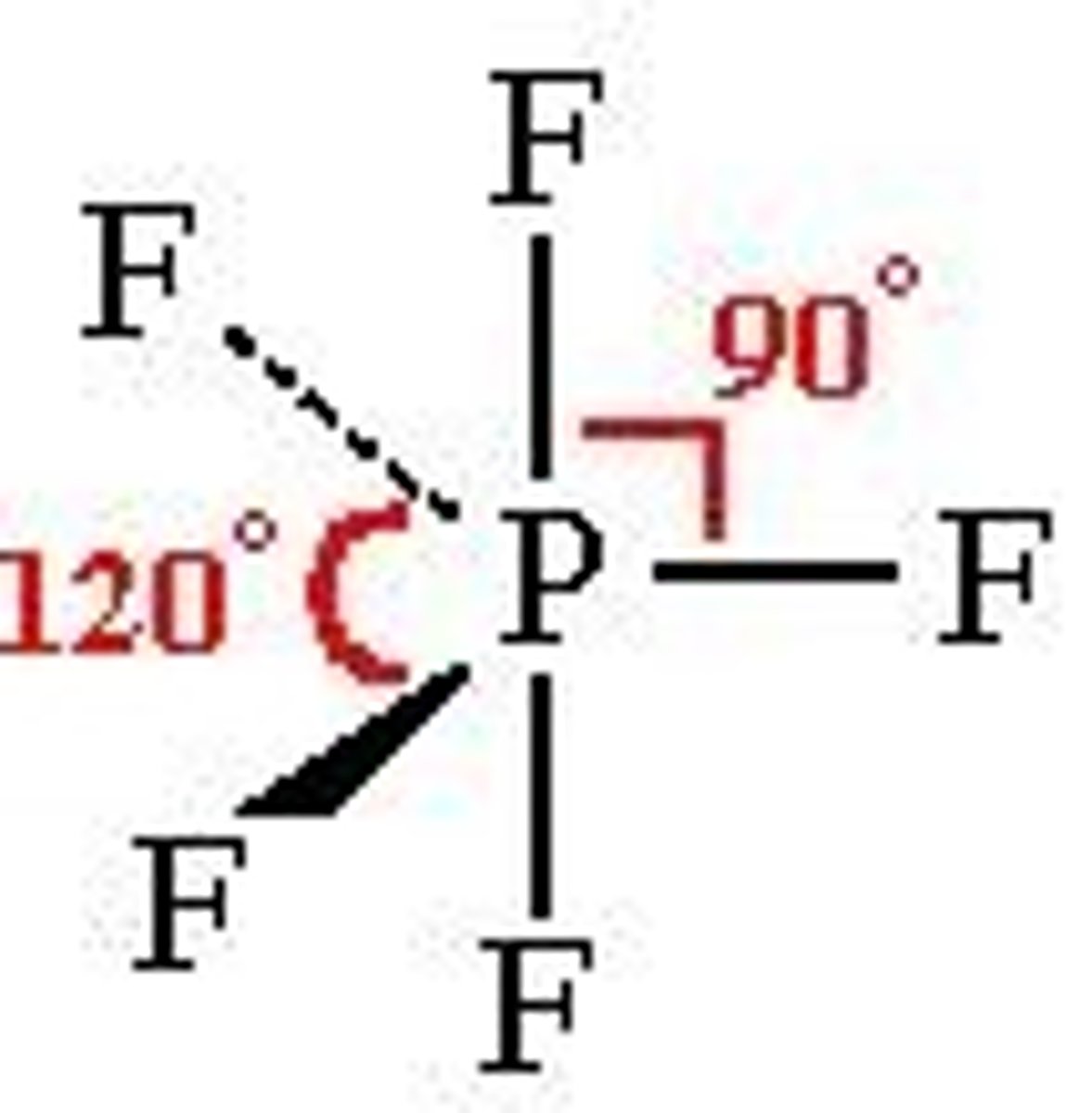
What is the Electron Geometry of PF5? Molecular Geometry?
Electron Geometry of PF5 is Trigonal Bipyramidal.
Molecular Geometry of PF5 is Trigonal Bipyramidal.
What is the Electron Geometry of XeF2? Molecular Geometry?
Electron Geometry of XeF2 is Trigonal Bipyramidal.
Molecular Geometry of XeF2 is Linear.
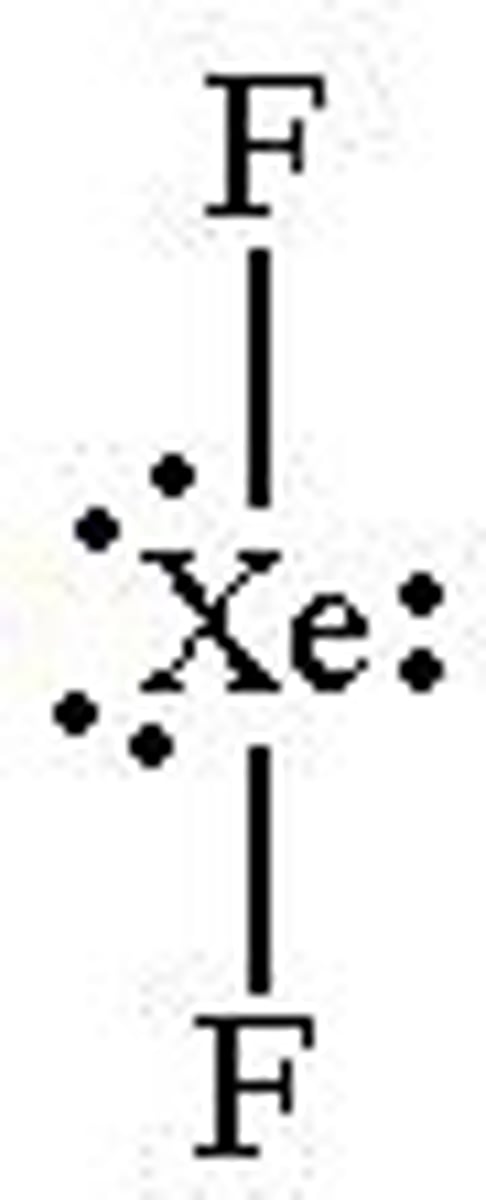
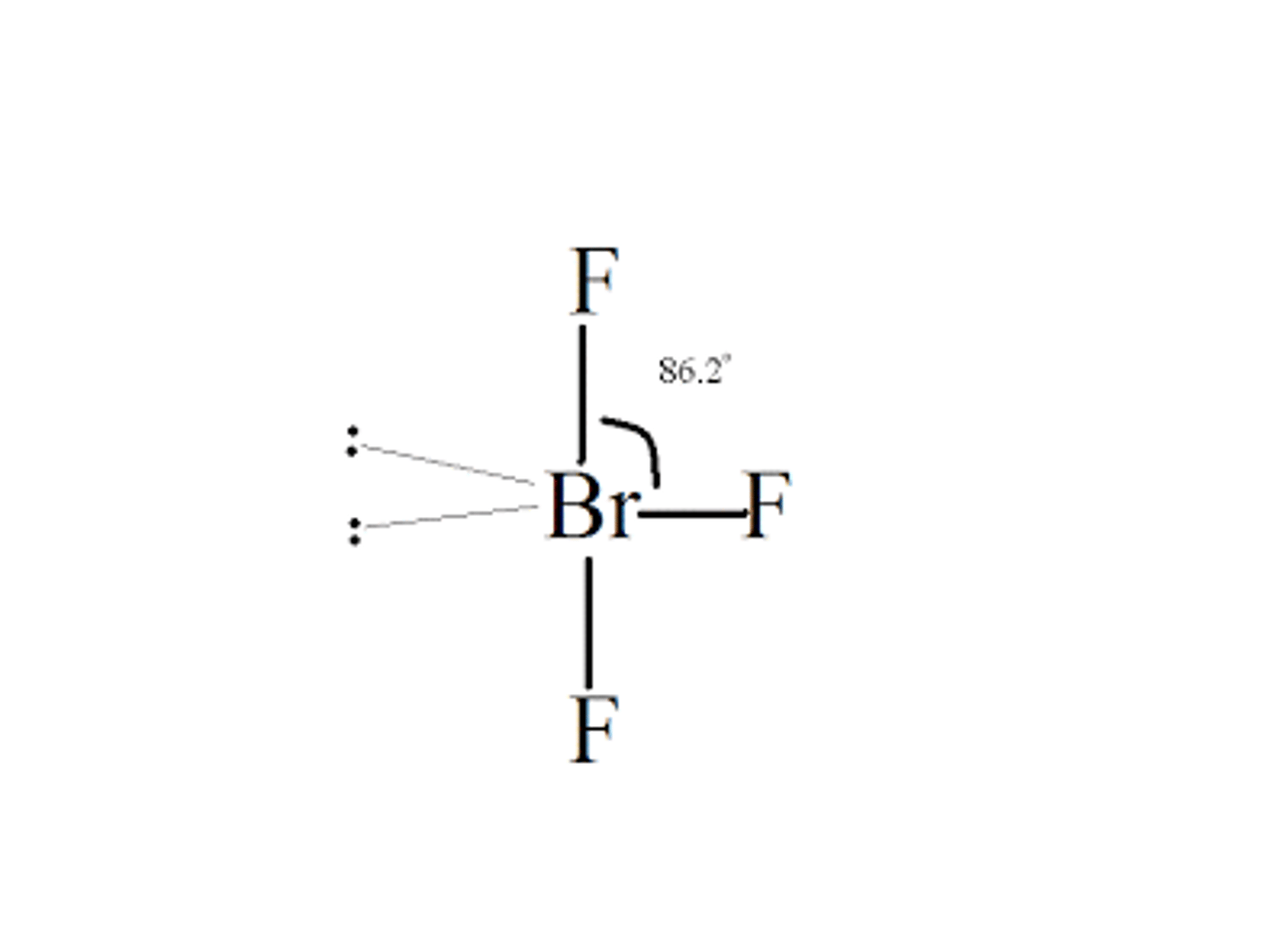
What is the Electron Geometry of BrF3? Molecular Geometry?
Electron Geometry of BrF3 is Trigonal Bipyramidal.
Molecular Geometry of BrF3 is T-shaped.
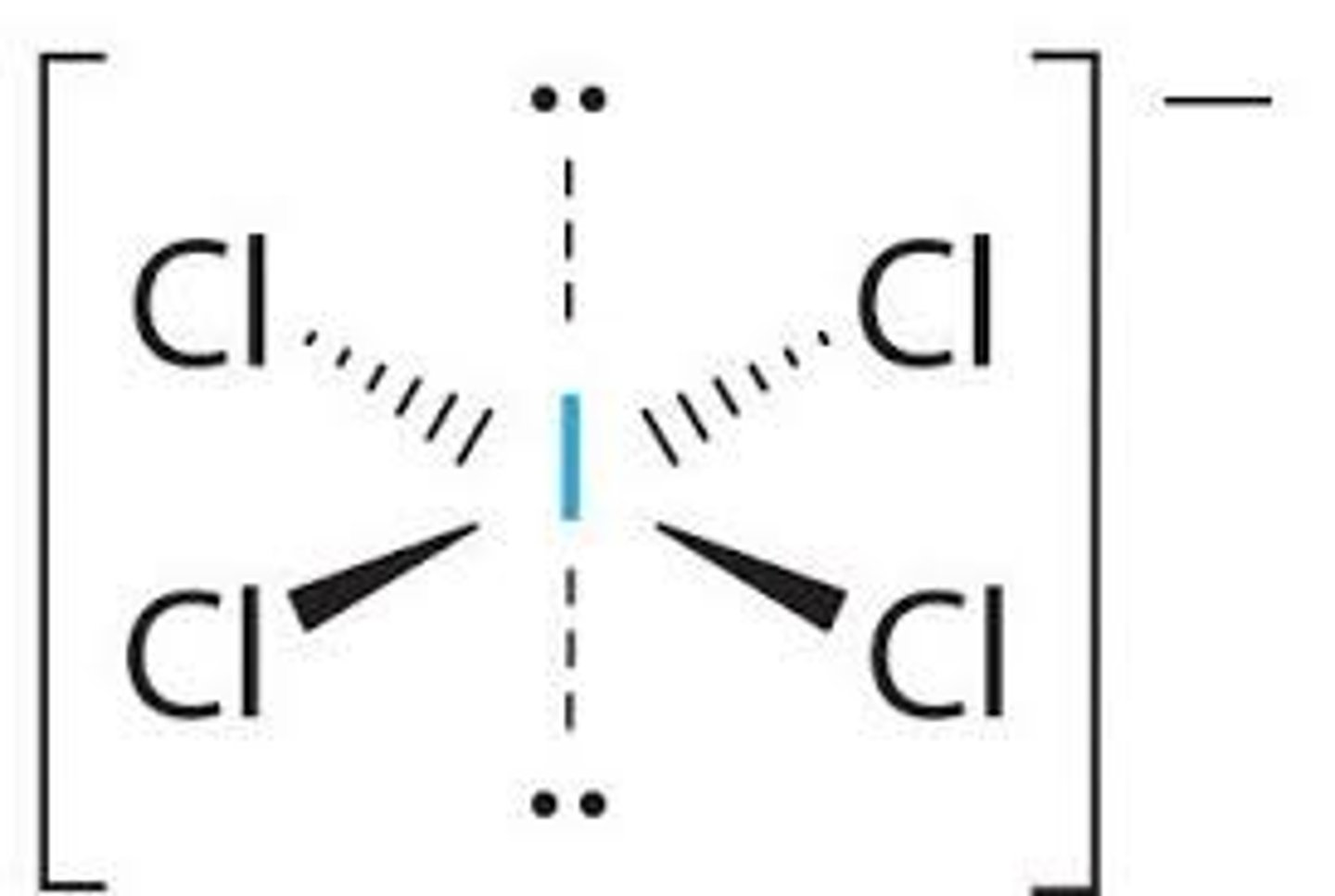
What is the Electron Geometry of ICl4-? Molecular Geometry?
Electron Geometry of ICl4- is Octahedral.
Molecular Geometry of ICl4- is Square Planar.
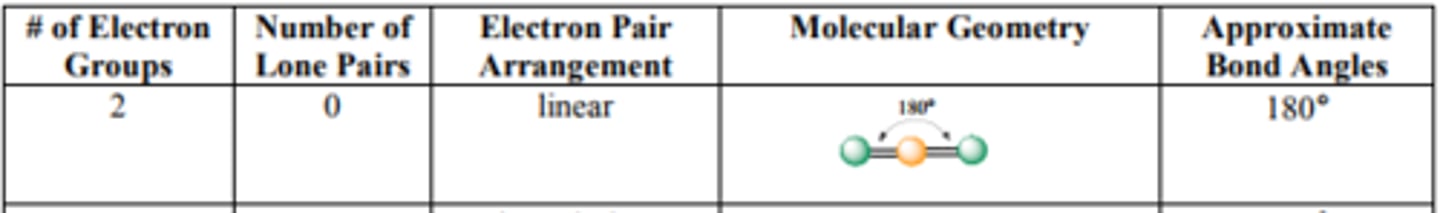
What are the bond angles for Linear?
Source: http://mmstcchemistry.weebly.com/uploads/2/4/1/2/24121933/chem162_moleculargeometry.pdf
What are the bond angles for Trigonal Planar? Bent?
Source: http://mmstcchemistry.weebly.com/uploads/2/4/1/2/24121933/chem162_moleculargeometry.pdf
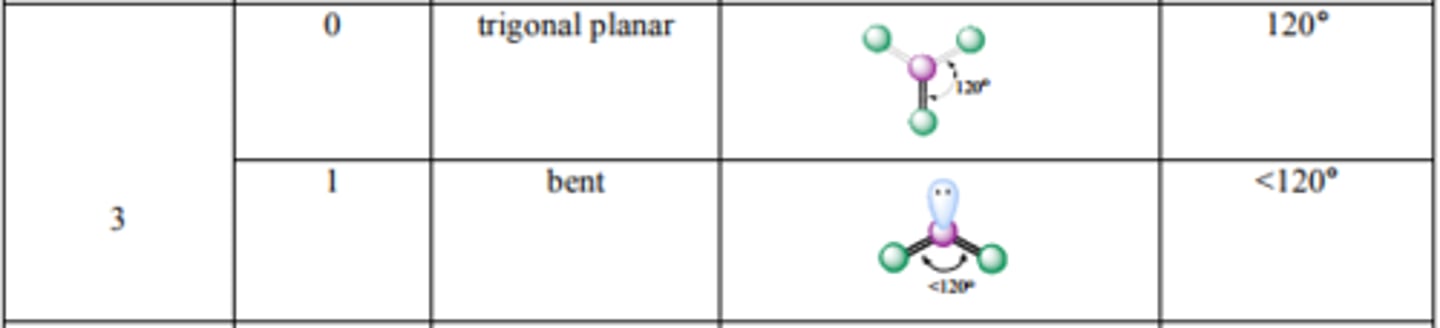
What are the bond angles for Tetrahedral? Trigonal Pyramidal? Bent?
Source: http://mmstcchemistry.weebly.com/uploads/2/4/1/2/24121933/chem162_moleculargeometry.pdf
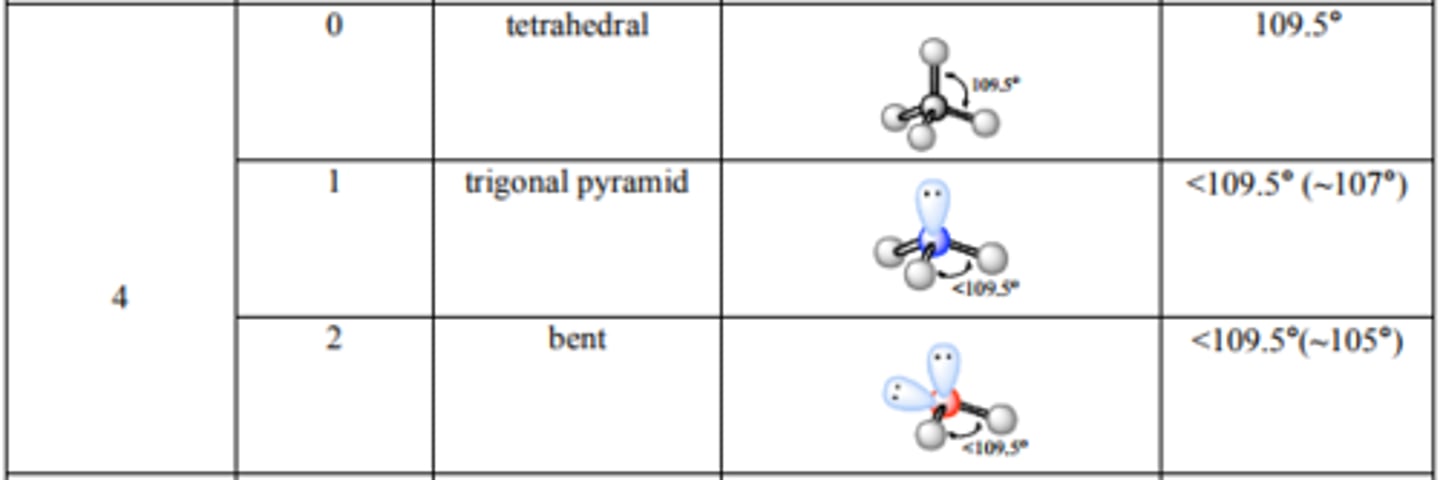
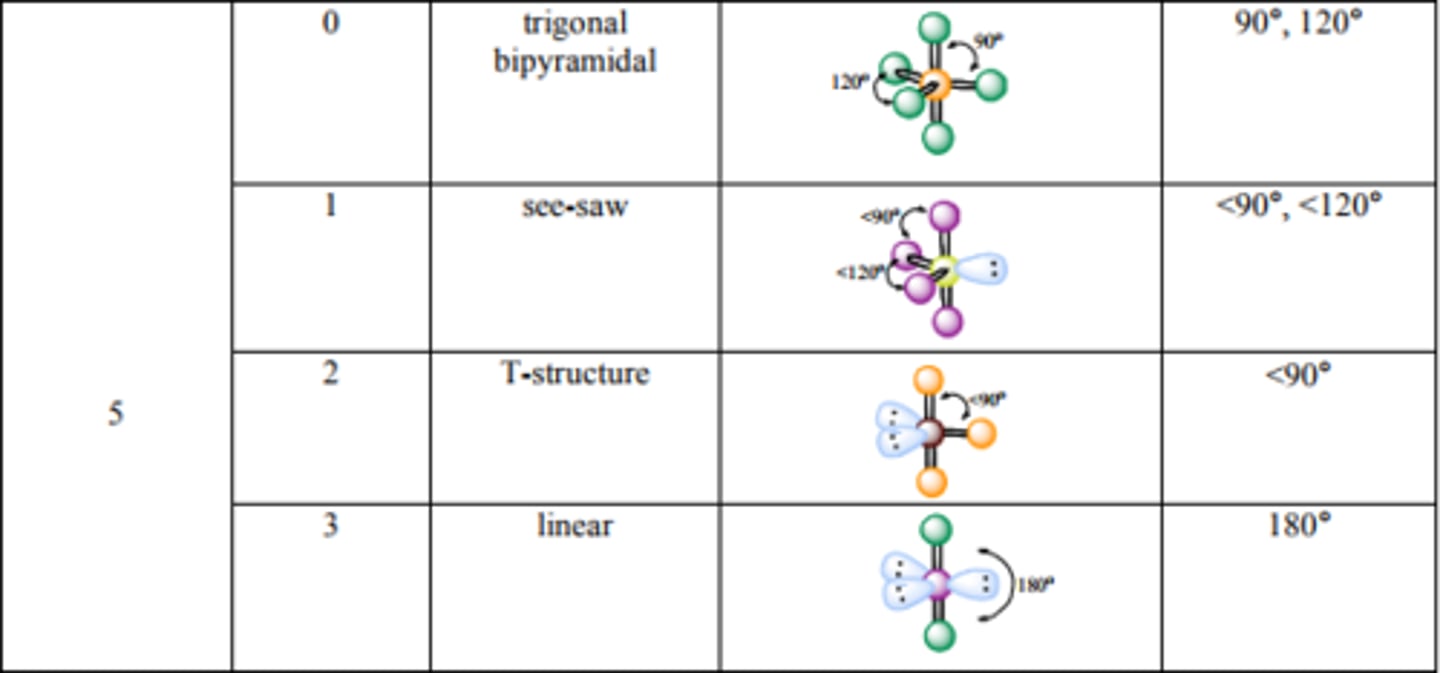
What are the bond angles for Trigonal Bipyramidal? See-saw?
T-structure? Linear?
Source: http://mmstcchemistry.weebly.com/uploads/2/4/1/2/24121933/chem162_moleculargeometry.pdf
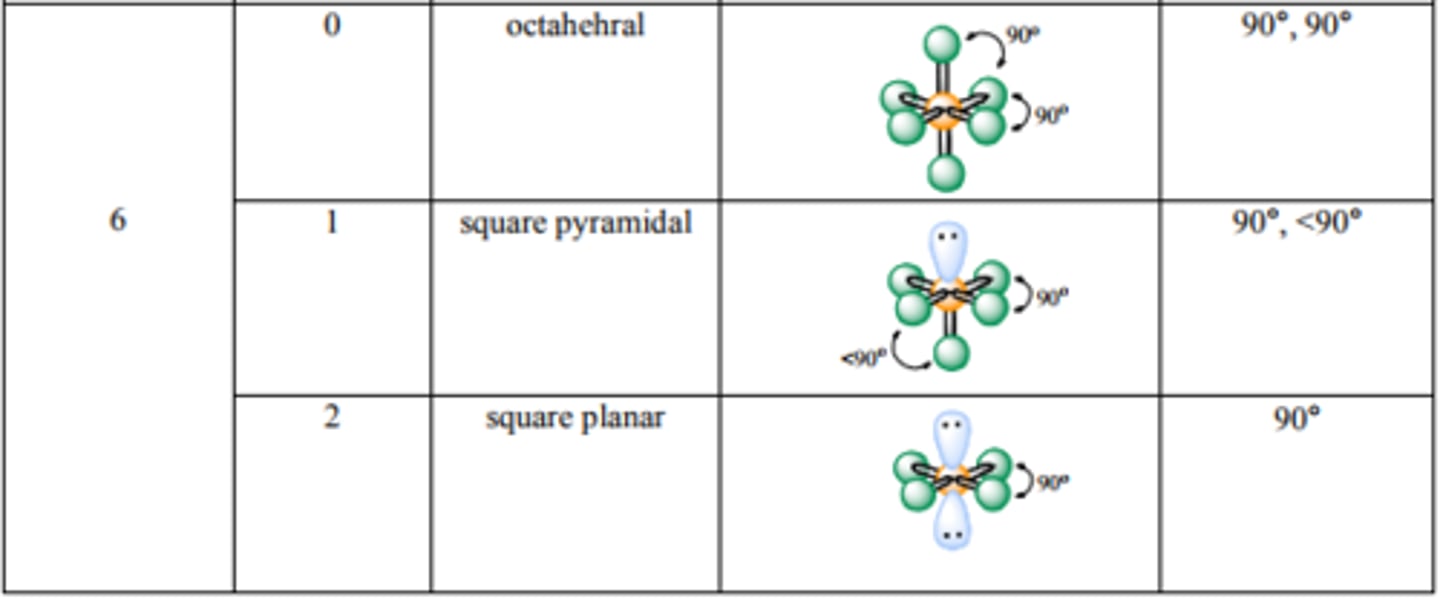
What are the bond angles for Octahedral? Square Pyramidal? Square Planar?
Source: http://mmstcchemistry.weebly.com/uploads/2/4/1/2/24121933/chem162_moleculargeometry.pdf
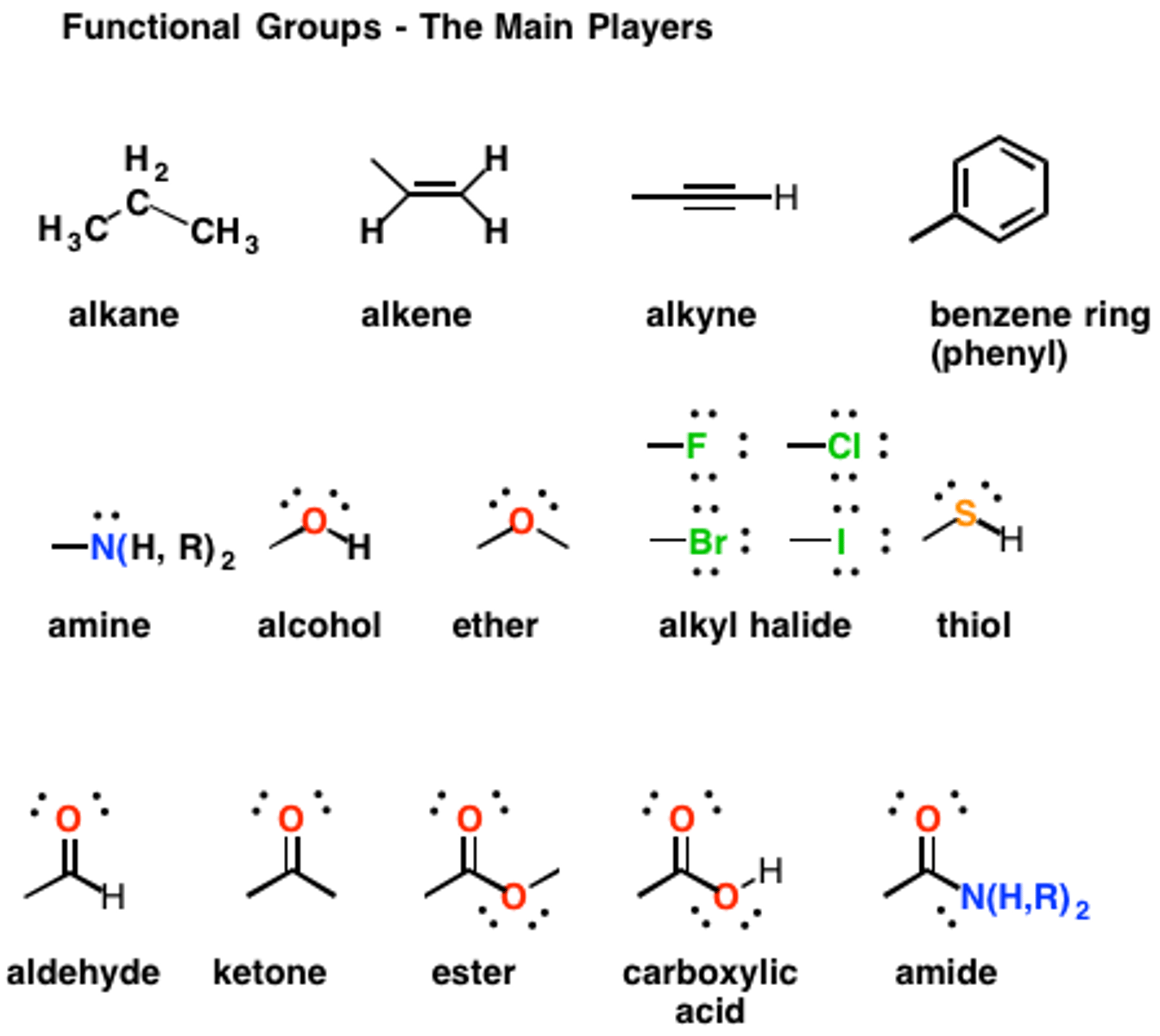
CRB What type of hybridization does a Carbon atom in an alkane have?
(A) sp
(B) sp2
(C) sp3
(D) sp4
(C) sp3
A Carbon atom in an alkane will have sp3 hybridization.
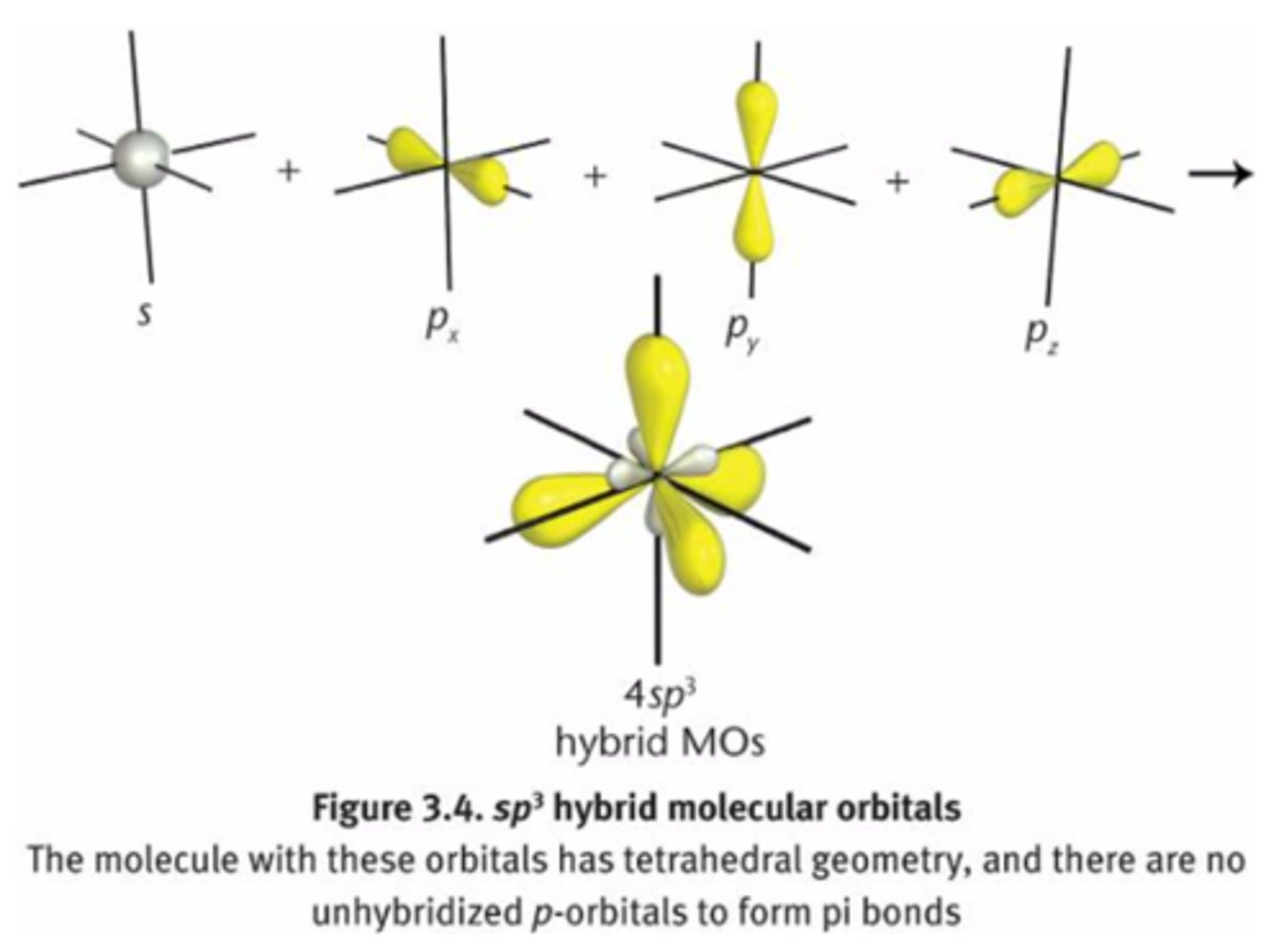
CRB Fill in the blanks: In sp3 hybridization, the hybrid molecular orbitals have ________ s character and _______ p character.
(A) 50%, 50%
(B) 33%, 67%
(C) 25%, 75%
(D) 20%, 80%
(C) 25%, 75%
In sp3 hybridization, the hybrid molecular orbitals have 25% s character and 75% p character.
This means that an sp3 orbital will closer resemble a p-orbital than an s-orbital
CRB Fill in the blanks: A ____ bond can form using hybridized orbitals, whereas a ____ bond must use a non-hybridized p-orbital.
(A) σ , π
(B) π , σ
(C) σ , θ
(D) θ , π
(A) σ , π
A σ bond can form using hybridized orbitals, whereas a π bond must use a non-hybridized p-orbital.
CRB Sometimes a p-orbital will not be hybridized, and therefore other orbitals will not have sp3 hybridization. Which of the following examples WOULD have sp3 hybridization?
(A) Alkene Carbon
(B) T-butyl central Carbon
(C) Alkyne Carbon
(D) Carbon with a Lone Pair
(B) T-butyl central Carbon
The T-butyl central Carbon will have four sp3 hybrid orbitals.
Alkenes and Alkynes have unhybridized p-orbitals to form pi bonds for double and triple-bonds, respectively.
A lone pair would not be hybridized ever.
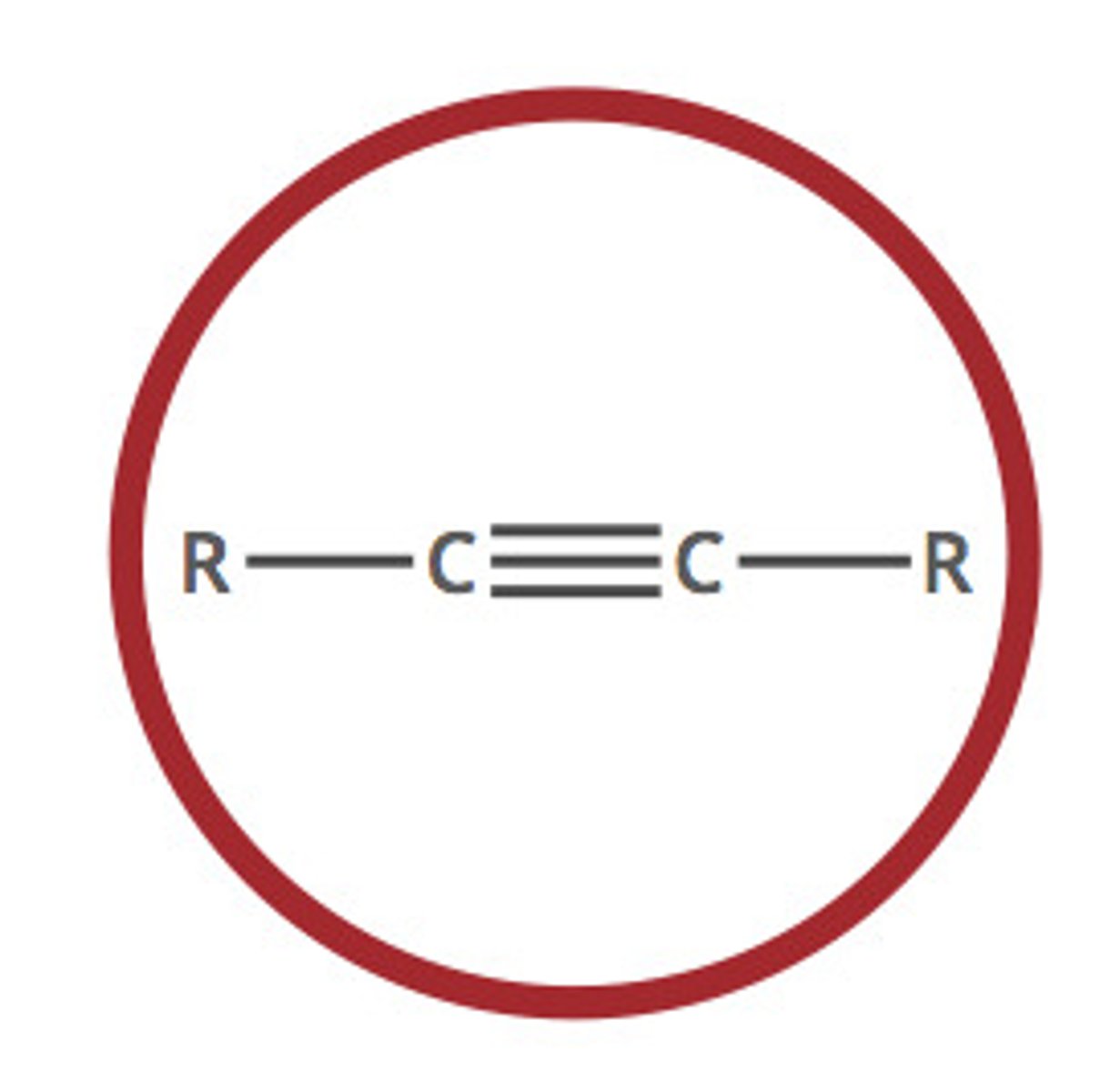
CRB As the bond order increases, the bond length decreases. So, if there were an enzyme that affected only bonds longer than a given length, which of the following would LEAST likely be affected?
(A) A hydrocarbon
(B) A C-F bond
(C) An alkene
(D) An alkyne
(D) An alkyne
This question is essentially asking which of the following bonds is shortest/ has the largest bond order.. An alkyne bond is a triple bond, meaning it has a bond order of 3. This is higher than the bond order for an alkene (2) or C-F or hydrocarbon (1).
CRB That alkyne in the previous questions has what type of hybridization of its molecular orbitals?
(A) sp
(B) sp2
(C) sp3
(D) sp5
(A) sp
For each Carbon, two p-orbitals must not hybridize so they can form pi-bonds. This leaves one s-orbital and one p-orbital to hybridize.
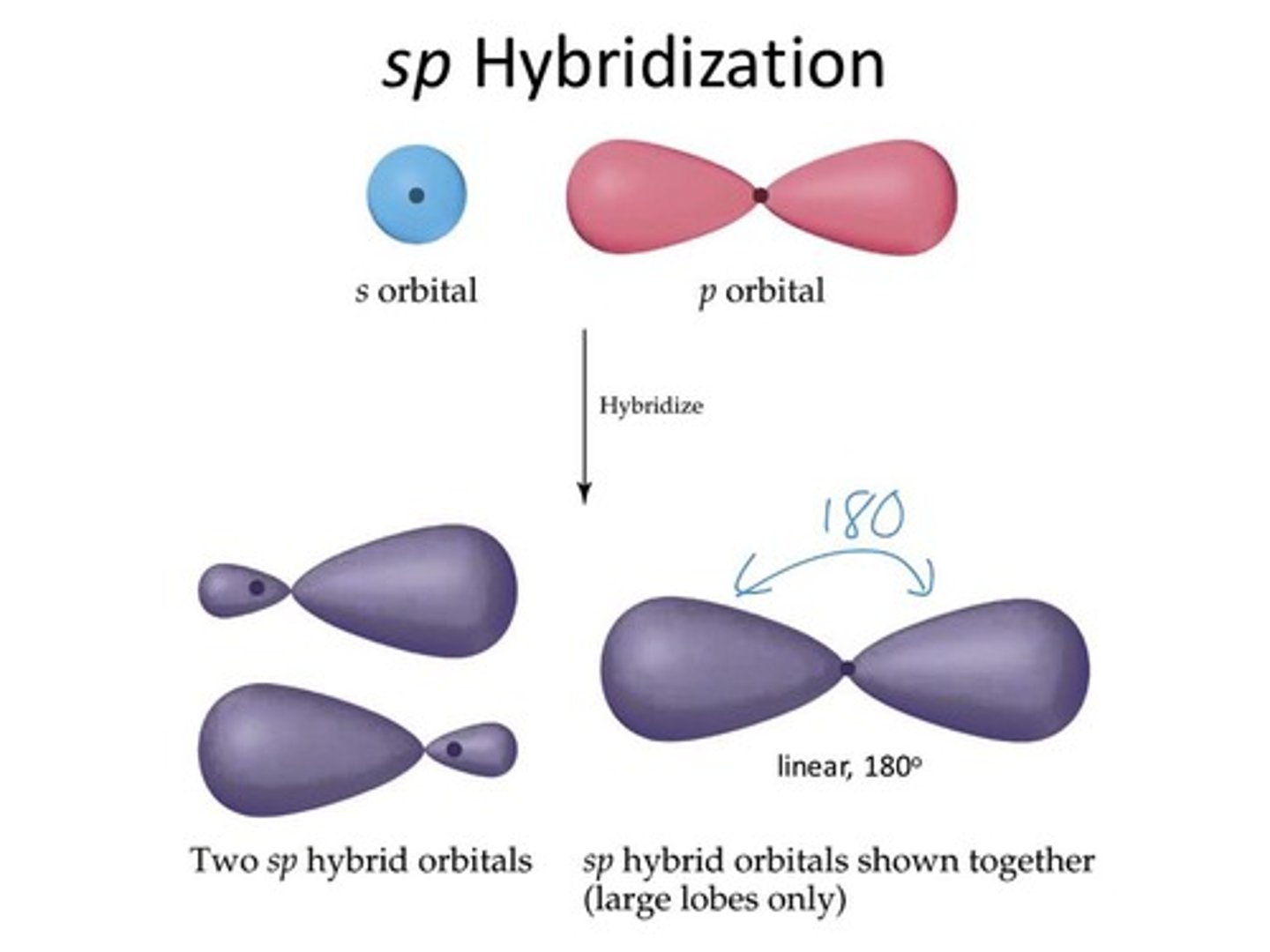
CRB Fill in the blanks: In sp hybridization, the hybrid molecular orbitals have ________ s character and _______ p character.
(A) 50%, 50%
(B) 33%, 67%
(C) 25%, 75%
(D) 20%, 80%
(A) 50%, 50%
In sp hybridization, the hybrid molecular orbitals have 50% s character and 50% p character.
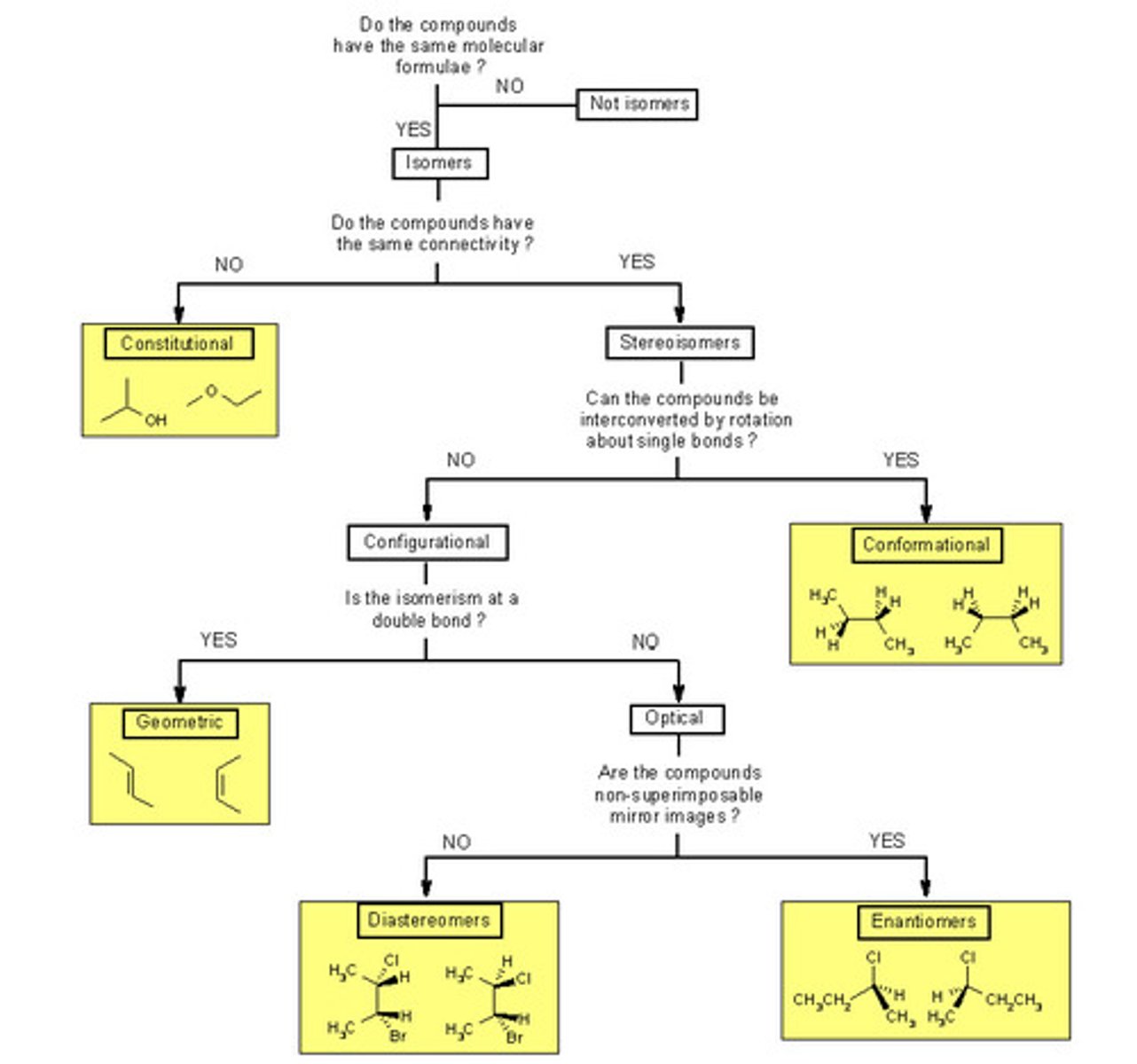
How are Constitutional Isomers similar and different from one another?
How are Stereoisomers similar and different from one another?
Constitutional Isomers have the same atomic makeup (molecular formula), but a different physical arrangement.
Stereoisomers have the same atomic makeup, but a different 3-D arrangement.
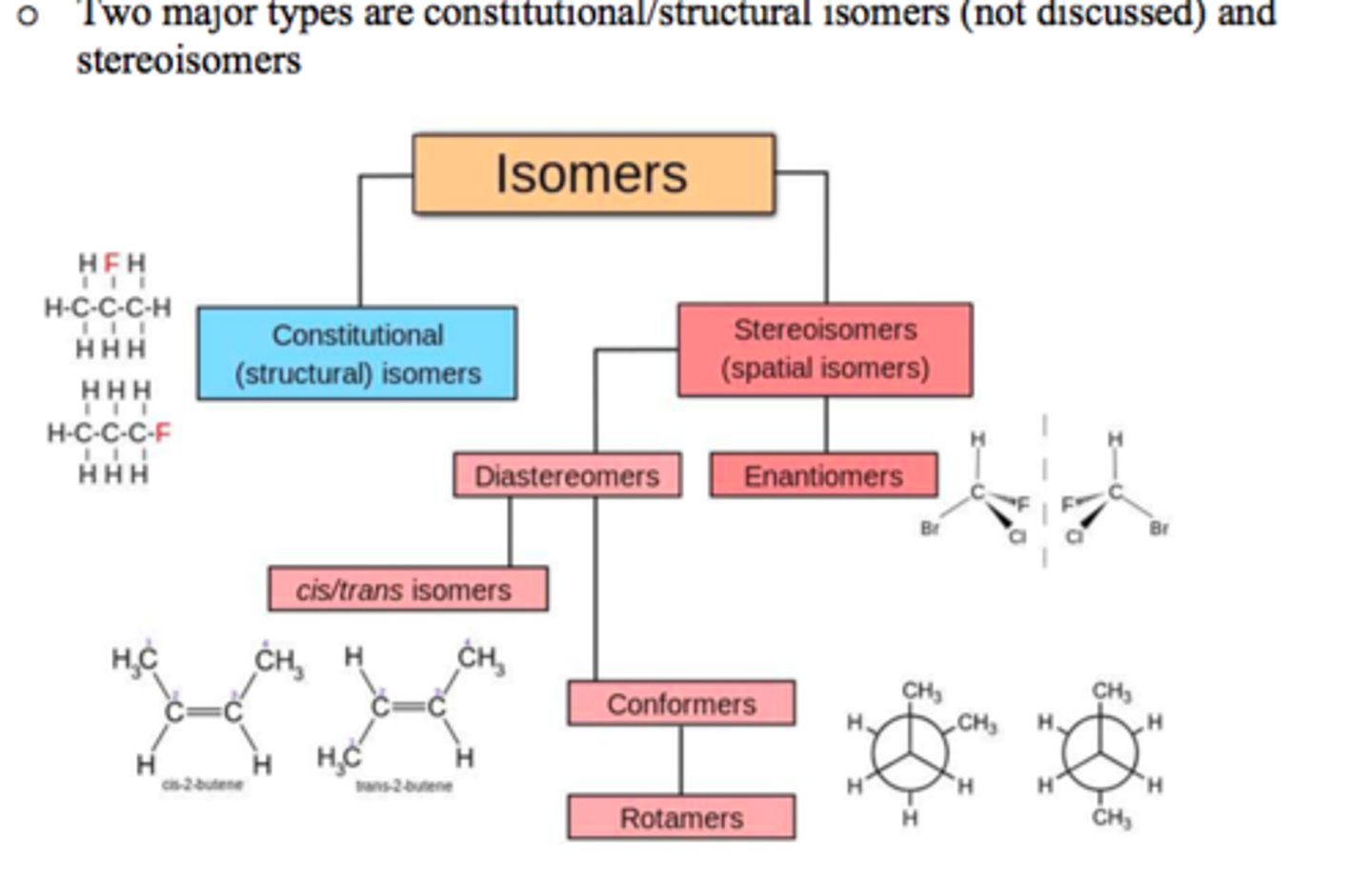
Enantiomers are an example of which of the following? What are Enantiomers?
(A) Constitutional Isomers
(B) Conformational Isomers
(C) Stereoisomers
(D) Geometric Isomers
(C). Stereoisomers
Enantiomers are an example of Stereoisomers, which are defined as non-superimposible mirror images.
Conformational isomers can be interconverted by only rotating bonds.
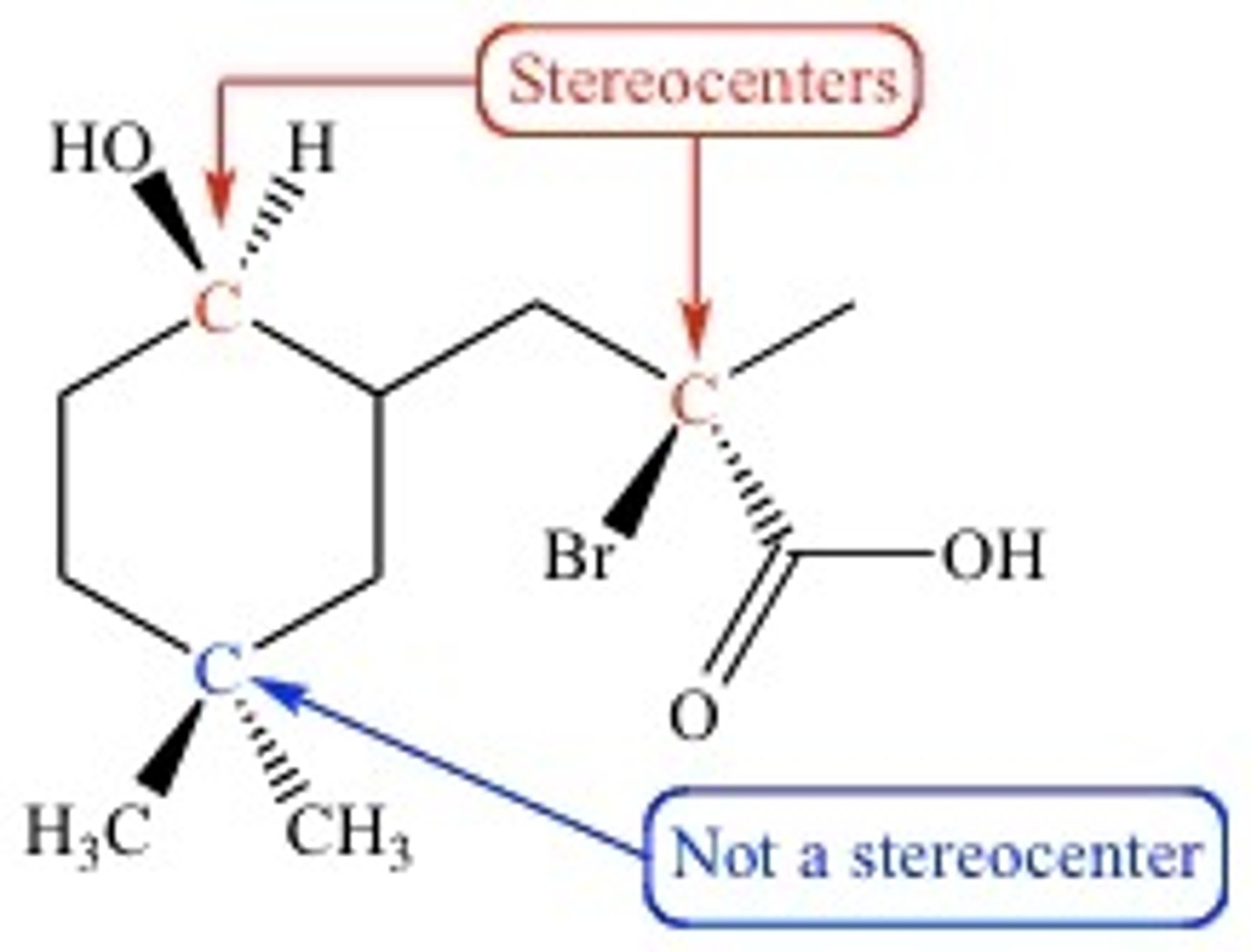
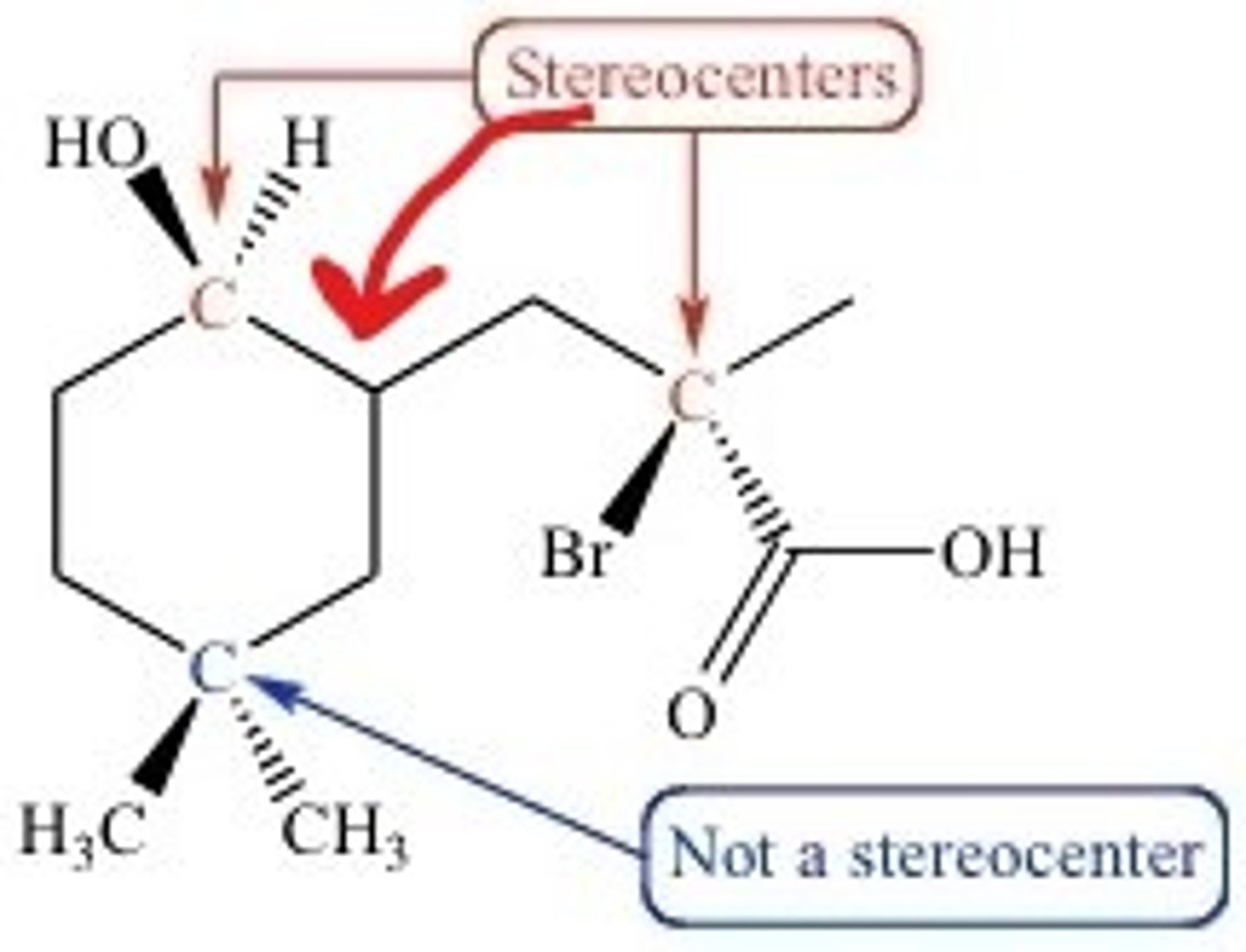
The center carbon of an enantiomer is called a Chiral/Chirality Center/Stereocenter. What is that?
A Stereocenter is a tetrahedral carbon with four different substituents.
CRB What equation tells you the number of possible stereoisomers?
#SI = 2^n
#SI = # of stereoisomers
n = number of stereocenters.
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
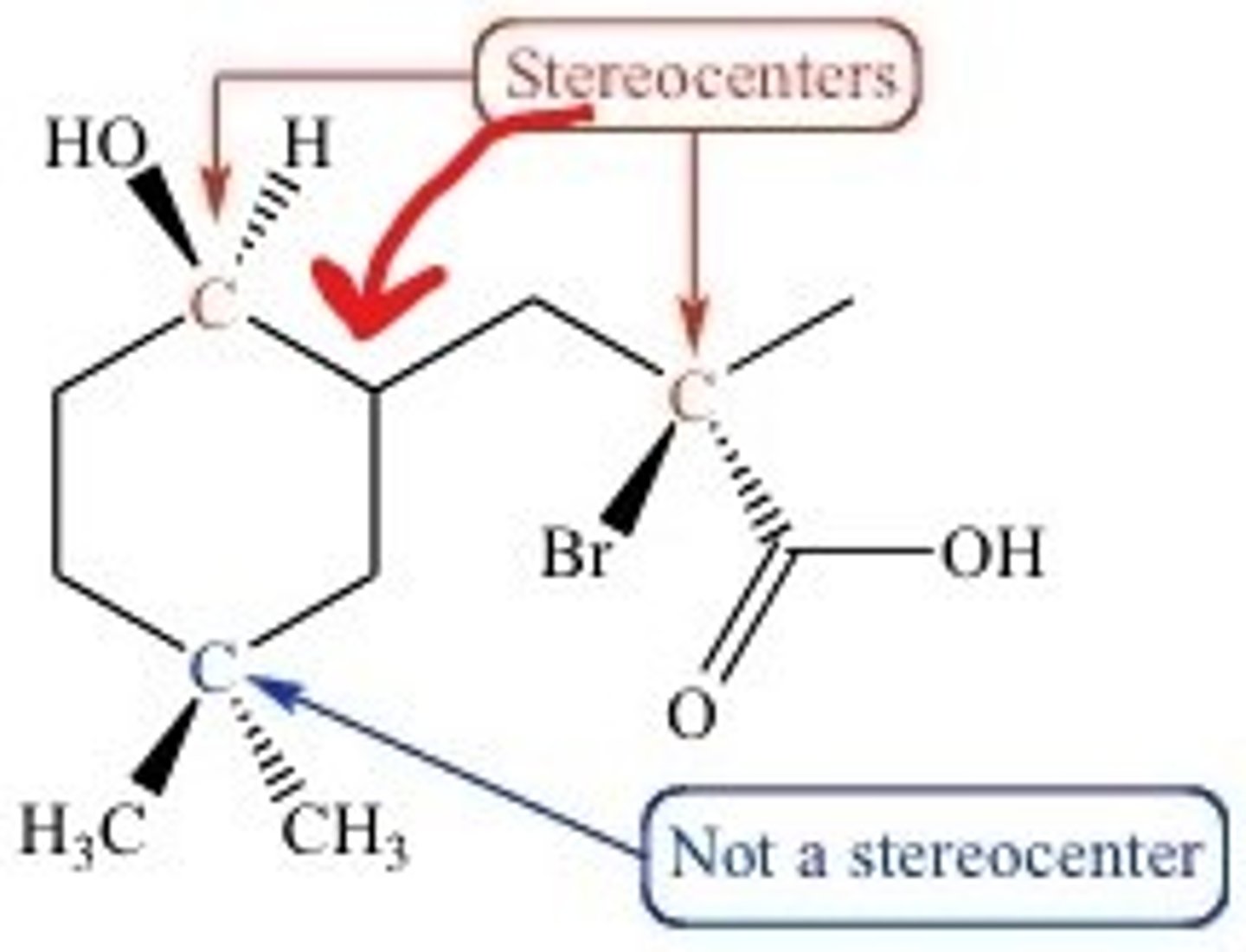
CRB The molecule on the previous notecard has how many possible stereoisomers?
(A) 1
(B) 2
(C) 4
(D) 8
(D) 8
#SI = 2^n
#SI = 2^3
#SI = 8
The molecule has 3 stereocenters; thus, it has 8 different possible stereoisomers.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB Which of the following intermolecular forces can be experienced by non-polar molecules?
(A) Dipole-Dipole interactions
(B) Hydrogen Bonding
(C) London Dispersion Forces
(D) Strong Nuclear Force
(C) London Dispersion Forces
London dispersion forces are a type of van der Waals force, and are caused by transient, induced dipoles.
CRB Dipole-Dipole interactions will try to keep the positive region of one molecule near the negative region of another. In which of the following states will these interactions be present and significant?
I. Solid
II. Liquid
III. Gas
(A) I only
(B) II only
(C) I and II only
(D) I, II and III
(C) I and II only
Dipole-Dipole interactions are mainly experienced by solids and liquids. In a gas, the molecules have an increased distance between each other and move faster, making Dipole-Dipole interactions negligible.
CRB Hydrogen bonds are one of the strongest intermolecular forces. Which of the following elements bound to Hydrogen would NOT create a Hydrogen Bond?
(for a periodic table, see http://www.sbcs.qmul.ac.uk/iupac/AtWt/table.gif )
(A) N
(B) S
(C) O
(D) F
(B) S
The three elements that, when bound to Hydrogen, can form Hydrogen bonds are Nitrogen, Oxygen and Fluorine.
CRB Based on the previous question, which of the following functional groups could NOT form a hydrogen bond?
(A) Thiol
(B) Fluorocarbon
(C) Hydroxyl
(D) Amine
(A) Thiol