Week 7: Precision Medicine
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
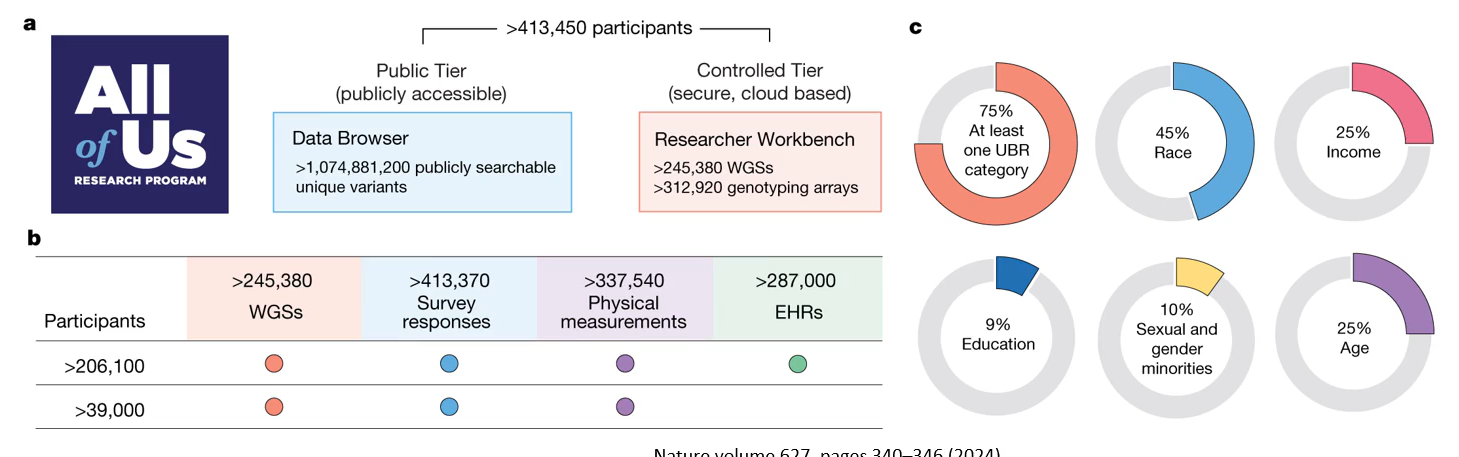
Underrepresented participants in genetic research
Genetic Information Nondiscrimination Act (GINA), 2008
Health Insurance (Title I):
Prohibits health insurers from discrimination based on the genetic information of enrollees
Info stays between you and physicians
May not use genetic information to determine is someone is eligible for insurance or to make coverage, underwriting or premium setting decisions
Can’t look and say “oh you're at risk.. So we won’t provide this”
May not request or require individuals or their family member to undergo genetic testing or to provide genetic information
GINA does not cover long-term care insurance, life insurance, or disability insurance.
Genetic Information Nondiscrimination Act (GINA), 2008
Employment (Title II):
Prevents employers from using genetic information in employment decisions such as hiring, firing, promotions, pay, and job assignments,
Prohibits employers or other covered entities (employment agencies, labor organizations, joint labor management training programs, and apprenticeship programs) from requiring or requesting genetic information and/or genetics tests as a condition of employment
The military is permitted to use genetic information to make employment decisions
Does not apply to employers with fewer than 15 employees
Q: What do federal regulations require before human subjects research can begin?
A: Federal regulations, such as those from the U.S. Food and Drug Administration and the Department of Health and Human Services (DHHS), require IRB review and approval before human subjects research can begin.
Q: Are the Nuremberg Code and the Declaration of Helsinki laws?
A: No — neither is a law. They are ethical guidelines, but many modern research laws and regulations are based on their principles.
Q (Nuremberg Code): Who may not be able to provide valid informed consent under the Nuremberg Code?
A: Babies, individuals with intellectual disabilities, and prisoners (due to possible coercion or limited capacity).
Q (Nuremberg Code): When was the Nuremberg Code created?
A: 1947.
Q: Why were early genomics databases not diverse?
A: They were populated mostly by Caucasians because recruitment ads were placed in newspapers and magazines primarily read by white people.
Q: Why are genomics programs now focusing on diversity?
A: To make databases more representative and useful for all populations.
Q: Why do some groups hesitate to participate in genomic research?
A: Due to past data abuses and mistrust of biomedical research.
Q: What are HeLa cells?
A: Cells taken from Henrietta Lacks without her consent; widely used in research.
Q: What is an example of unethical use of Indigenous biological samples?
A: Native tribes consented to diabetes research, but their samples were later used to study schizophrenia without consent.
Q (Nuremberg Code): What historical event led to the creation of the Nuremberg Code?
A: Nazi doctor abuses during WWII.
Q (Nuremberg Code): What is the core principle of the Nuremberg Code?
A: Voluntary consent of human subjects.
Q (Nuremberg Code): What must occur before a study is done on humans?
A: Animal studies or other prior scientific research.
Q (Nuremberg Code): Should humans ever be the first test subjects?
A: No — humans should not be used as first subjects.
Q (Nuremberg Code): What must researchers avoid in experiments?
A: Unnecessary physical or mental suffering.
Q (Nuremberg Code): When should research not move forward?
A: If death or permanent injury is expected.
Q (Nuremberg Code): What must researchers assess before conducting research?
A: A risk-versus-benefit analysis.
Q (Nuremberg Code): What should be prepared before the experiment begins?
A: Protections and safeguards for participants.
Q (Nuremberg Code): What must researchers do if they foresee potential harm?
A: Have a plan to handle adverse effects.
Q (Nuremberg Code): Who is allowed to conduct human research?
A: Only qualified and skilled researchers.
Q (Nuremberg Code): What rights do participants have during a study?
A: They can opt out at any time.
Q (Nuremberg Code): What must researchers do if the study becomes dangerous?
A: Immediately terminate the experiment.
Q (Helsinki): What does the Declaration of Helsinki establish?
A: Ethical principles for clinical research involving humans.
Q (Helsinki): When was the Declaration of Helsinki adopted?
A: Between 1961–1964.
Q (Helsinki): What is a key ethical requirement under Helsinki?
A: Informed consent.
Q (Helsinki): What must research be based on before involving humans?
A: Animal models or preclinical studies.
Q (Helsinki): Who may conduct research according to Helsinki?
A: Only qualified researchers.
Q (Helsinki): What analysis must be performed before conducting a study?
A: A risk-benefit (cost-benefit) analysis.
Q (Helsinki): When is extra justification required under Helsinki?
A: When asking healthy volunteers to take risks for the “greater good.”