2: Mitochondrial Bioenergetics
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Cytochrome c
a) Reduces cytochrome c1
b) Oxidises complex IV
c) Has a higher E’° than cytochrome aa3
d) Has one heme covalently bound to apoprotein
e) Is localised in the matrix
d) Has one heme covalently bound to apoprotein
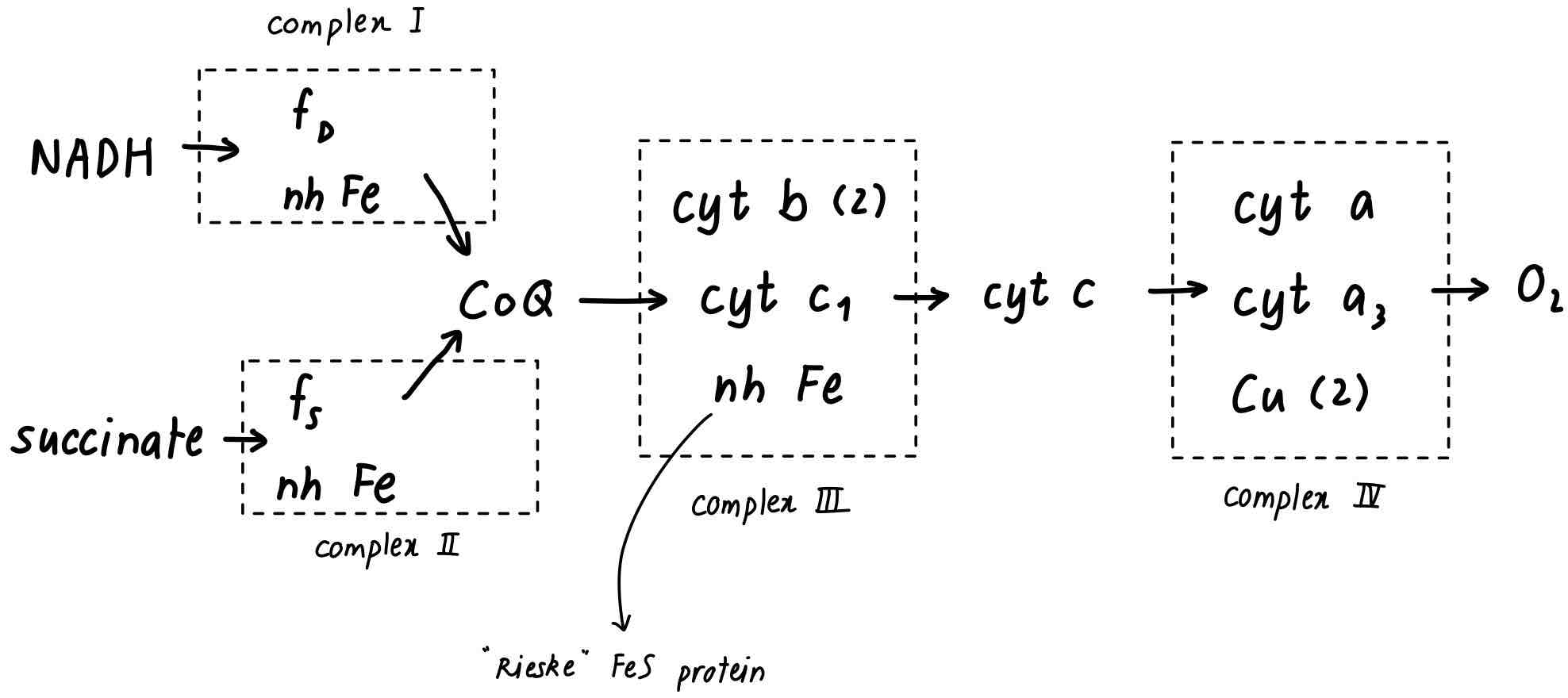
Cytochrome c1 is the redox centre in complex III (ubiquinol cytochrome c reductase) that acts as direct electron donor to cytochrome c. This it is cytochrome c1 that reduces cytochrome c.
a) False. Gets reduced by cytochrome c1
b) False. It reduces complex IV
c) False. The reason it reduces complex IV is because of its lower E’°
d) True. Hemes c are characterised by covalent binding of the vinyl groups to SH in the apoproteins. This is also true for cytochrome c1
e) False. It is localised in the intermembrane space
Cytochrome c can be extracted by saline solution after osmotic shock of mitochondria, but not of submitochondrial vesicles that have an inverted sidedness with respect to mitochondria. Explain.
By applying ultrasonic irradiation to mitochondria, cristae are pinched out, forming small vesicles that have an opposite orientation with respect to intact mitochondria.
Cytochrome c is located on the outer surface of the inner membrane, so after an osmotic shock that breaks down the outer membrane, it is released in the aqueous medium.
On the other hand, in submitochondrial vesicles, cytochrome c is located in the inner surface and cannot be released in the external medium, since the inner medium is impermeable to proteins
During apoptosis, cytochrome c is released in the cytosol, but Krebs cycle enzymes are not. What can you deduce about the localisation of these proteins?
a) Cytochrome c is loosely bound to the membrane whereas Krebs cycle enzymes are tightly bound
b) Cytochrome c is loosely bound on the external face of the inner membrane, whereas Krebs cycle enzymes are in the matrix
c) Cytochrome c is small enough to cross the inner membrane
d) Krebs cycle enzymes form an insoluble complex
b) Cytochrome c is loosely bound on the external face of the inner membrane, whereas Krebs cycle enzymes are in the matrix
During apoptosis, the outer membrane becomes permeable to proteins and cytochrome c is released in the cytosol. On the other hand, Krebs cycle enzymes are localised in the matrix, so they remain in situ after permeabilization of the outer membrane.
NAD+ reduction by succinate:
succinate + NAD+ → fumarate + NADH + H+
a) Occurs spontaneously (is exergonic)
b) Requires energy because [NAD+] is low
c) Requires energy because E° [NAD+][NADH] is more negative than E° [fumarate][succinate]
d) Requires energy because E° [NAD+][NADH] is less negative than E° [fumarate][succinate]
c) Requires energy because E° [NAD+][NADH] is more negative than E° [fumarate][succinate]
The redox couple [NAD+][NADH] is much more negative than the couple [fumarate][succinate]. Thus, the reaction may occur spontaneously in the opposite direction. The reduction of NAD+ by succinate then requires energy in the form of membrane potential
During respiration
a) The matrix becomes more negative and more alkaline
b) The matrix becomes more negative and more acidic
c) The matrix becomes more positive and more acidic
d) The matrix becomes more positive and more alkaline
e) No change occurs in the matrix
a) The matrix becomes more negative and more alkaline
During respiration, H+ are ejected from the matrix to the intermembrane space. H+ accumulate out of mitochondria and the external space becomes more acidic and more positive, whereas the matrix becomes more alkaline and more negative
Only one is wrong. If we inhibit respiratory chain with Antimycin A:
a) CoQ becomes more reduced
b) Cytochrome c becomes more oxidised
c) Cytochrome a becomes more reduced
d) NAD+ is reduced to NADH
e) The consumption of O2 strongly decreases
c) Cytochrome a becomes more reduced
Antimycin A is a classical inhibitor of Complex III. After inhibition, respiration from NADH and succinate ceases. We expect that, in the presence of substrates and oxygen, components downhill of Complex III become more oxidised. So CoQ becomes more reduced.
a) True. Cytochrome c will therefore become more oxidised
b) True.
c) False. Cytochrome a is in Complex IV, downhill of the inhibition step
d) True.
e) True. Electrons do not flow to oxygen anymore
Glycerol-P is oxidised by CoQ in the respiratory chain. What is the ATP yield when the final acceptor is oxygen?
a) 2.5 ATP / 2e-
b) 2.0 ATP / 2e-
c) 1.5 ATP / 2e-
d) 1.0 ATP / 2e-
e) 0.5 ATP / 2e-
c) 1.5 ATP / 2e-
Glycerol-P is oxidised by mitochondrial Glycerol-P dehydrogenase that has CoQ as acceptor. Thus, electron transfer from glycerol P follows the same pathway as that from succinate (1.5 ATP / 2e-).
If we add succinate to isolated mitochondria, we obtain v = 0.1 µmol/min mg
Adding ADP, v = 0.75 µmol/min mg
Calculate the RCI
What rate is presumably obtained by adding oligomycin, DNP or KCN, respectively?
RCI = 7.5
RCI = v state 3 (ADP) / v state 4 (no ADP) = 0.75/0.1 = 7.5
Oligomycin: blocks ATP synthesis, so rate goes back to state 4
DNP: If this uncoupler is added in State 3, the rate may increase further because the membrane permeability may exceed the capacity of ATP synthase proton channel
KCN: Inhibits Complex IV. Rate tends to 0
Surprisingly, a mitochondrial preparation respiring with succinate has high rate that is not enhanced by ADP. What may be the reason?
a) ATP synthase is inhibited (e.g. by contaminants in medium)
b) Mitochondria are uncoupled (e.g. membrane is damaged)
c) Concentration of succinate is too high
d) Concentration of O2 is too low
b) Mitochondria are uncoupled (e.g. membrane is damaged)
If a mitochondrial preparation respires in absence of ADP, we must presume it is uncoupled: uncoupling in absence of protonophores may simply be because the inner membrane has been damaged during preparation and purification of mitochondria. Damaged membranes become leaky to protons, so the membrane potential is collapsed.
a) False. Inhibition of ATP synthase would decrease the rate (in coupled mitochondria)
c) Substrate concentration is usually saturating, so an eventual decrease below Km could actually decrease the rate
d) False. Low O2 could only decrease rate
It was suggested to use DNP to lose weight. Why?
Dinitrophenol is an uncoupler of oxidative phosphorylation that enhances oxygen consumption by dissipating the membrane H+ potential. Respiratory substrates are therefore oxidised at high rate without ATP synthesis. In this situation, oxidative metabolism is stimulated: high concentration of oxidised NAD+ stimulates Krebs cycle and fatty acid oxidation enzymes, with high consumption of fat; oxidation energy cannot be used for anabolism and is mostly released as heat.
Why does ROS generation increase at high mitochondrial membrane potential?
A high mitochondrial membrane potential is present in State 4, when H+ translocation to the exterior is low and most electron carriers in the respiratory chain are more reduced due to the pressure of substrates oxidation. ROS are oxygen molecules partially reduced by redox centres in Complex I, III and others; if the electron donors to oxygen are more reduced, ROS generation will increase.
Explain why calcium is transported inside mitochondria exploiting the membrane potential
The mitochondrial membrane potential is negative inside; thus cations may be transported inside if there is a suitable carrier; Ca2+ has a uniporter in the inner membrane and therefore is carried to the matrix during respiration
In the cytosol of rat hepatocytes the ratio [ATP]/[ADP][Pi] is 6×102. Calculate the free energy required to synthesise ATP in rat hepatocytes
47.2 kJ/mol
ΔG = ΔG’° + 6 log [ATP] / [ADP] [Pi]
ΔG = 30.5 + 6 log 600 = 47.2 kJ/mol
Calculate ΔE and ΔG for the total reaction of the respiratory chain, from NADH to O2 with [NAD+] = 100 mM and [NADH] = 1 mM (O2 at standard condition)
ΔE = -0.20 V, ΔG = -195 kJ/mol
E’° (NAD+/NADH) = -0.32 V
E’° (O2/H2O) = +0.81 V
E for the given conditions:
E = -0.32 + 0.06 log (10-1)/(10-3) = -0.20 V
ΔE for overall reaction = +0.81 - (-0.20) = +1.01 V
ΔG = -nFΔE (F = 96.5 kJ/V, n = 2) → ΔG = -96.5×1.01×2 = -195 kJ/mol
In a living cell, it is difficult to find mitochondria that respire under State 4 conditions. Why?
In a living cell, ATP is normally consumed to accomplish work, with formation of ADP. ADP stimulates respiration promoting State 3 in order to synthesise new ATP molecules.
Atractyloside is a drug that inhibits the adenine nucleotide translocator. What do you think may be the effect of its addition on respiration?
The adenine nucleotide translocator (ANT) is required to export synthesised ATP in exchange with cytosolic ADP. If we block ANT with atractyloside, ADP cannot enter in the matrix anymore and respiration is maintained in State 4. The effect is superimposable to that of oligomycin.
Why ATP synthase works as a proton pump (H+ ejected from matrix to cytosol) in presence of an uncoupler?
Keep in mind that the adenine nucleotide carrier may work in reverse (ATP enters)
Uncouplers dissipate the membrane potential, so that the concentration of H+ is equilibrated. Under this condition, ATP synthesised in the cytosol by glycolysis enters in the matrix by reverse action of ANT and energises ATP synthase as a H+ pump, ejecting H+ from matrix to outer medium
True or False?
In mitochondria, the proton-motive force is mostly due to pH difference
During coupled respiration, the matrix becomes more acidic
Uncouplers induce collapse of the H+ gradient
If we add an uncoupler to retenone-inhibited mitochondria, respiration is resumed
The respiratory control index is the ratio mol ATP formed/mol O2 consumed
Complex III is the largest respiratory complex in mitochondria
False. Due to buffering capacity, the proton-motive force in mitochondria mainly consists in a difference of membrane potential (Δψ)
False. Since H+ are removed from the matrix, it will become more alkaline
True. They are protonophores, thus they equilibrate H+ across the membrane.
False. If the respiratory chain is inhibited, this effect is upstream with respect to proton equilibration, thus an uncoupler will have no effect
False. This is the ADP/O2 ratio. The RCI is the ratio of respiration is State 3 (with ADP) / State 4 (without ATP)
False. The largest complex is Complex I
True or False? In the mitochondrial respiratory chain
FAD is a prosthetic group in Complex I
Complex III is a proton pump
CoQ is a mobile component between Complex III and Complex IV
The heme group of cytochrome b is covalently bound to apoprotein
Complex III contains copper
The E’° of cytochrome a is lower than that of cytochrome c
False. It is FMN
True. So are Complex I and Complex IV
False. It is the mobile complexes between Complex I or II and Complex III
False. The heme of cytochrome c is covalently bound to apoprotein
False. Complex IV contains copper
False. This would be against the direction of electron flow
Only one answer is false. Concerning the respiratory chain:
a) Uncouplers decrease the rate of electron transfer
b) Uncouplers decrease the rate of ATP synthesis
c) Cyanide inhibits Complex IV
d) CO inhibits Complex IV
e) Complex I pumps 4H+/2e- from matrix to IMS
a) Uncouplers decrease the rate of electron transfer
a) False. Uncouplers dissipate membrane potential by acting as protonophores in the membrane and increase the rate of electron transfer. At the same time, H+ re-entry is not coupled to ATP synthesis.
b) True
c) True
d) True. CO inhibits Complex IV by binding in the place of O2
e) True
Ferricyanide may accept electrons from cytochrome c in intact mitochondria.
In the presence of KCN (to inhibit Complex IV), state the proton translocation stoichiometry of NADH ferricyanide reductase and succinate ferricyanide reductase.
What are the corresponding ATP/2e- ratios?
Complex I pumps 4H+/2e-, Complex III pumps 4H+/2e-, ATP synthesis requires 4H+/ATP
NADH to ferricyanide: 8H+ translocated to outer space
Succinate to ferricyanide: 4H+ translocated
ATP/2e- (NADH) = 8/4 = 2
ATP/2e- (succinate) = 4/4 = 1
Concerning ATP synthesis
a) At the molecular level, formation of the anhydride bond of ATP does not require energy
b) The catalytic sector of ATP synthase protrudes in the intermembrane space
c) Oligomycin inhibits the catalytic activity of F1 by binding the catalytic site
d) Each ATP synthase has 6 β-subunits
e) The c subunits of F0 form a ring of 10 monomers in all different species
f) In humans the c subunit is encoded by mitochondrial DNA
a) At the molecular level, formation of the anhydride bond of ATP does not require energy
a) True. Energy is required to open the ATP site and release ATP
b) False. It protrudes in the matrix
c) False. Oligomycin inhibits the H+ channel of F0
d) False. There are 3 α and 3 β subunits
e) False. The c oligomer has different number of subunits in different species (8-12)
f) False. In humans the c subunit is encoded by nuclear DNA
Death by cyanide poisoning is due to tissue asphyxia, mainly in the central nervous system. Explain the reason and project a possible therapy considering that cyanide has higher affinity for metahaemoglobin than for cytochrome oxidase.
Since KCN inhibits respiration, most human cells die because they need aerobic oxidative phosphorylation. If an aliquot of blood is treated with an oxidizing agent, Hb(Fe2+) is oxidized to meta-Hb (Fe3+). After transfusion, the meta-Hb will bind KCN in competition with Complex IV of mitochondria.
FAD
a) Derives from Vitamin B6
b) Is a prosthetic group of Complex I
c) Is a prosthetic group of Complex II
d) Is a one-electron carrier only
e) Is found only in mitochondria
c) Is a prosthetic group of Complex II
a) False. FAD derives from vitamin B2 (riboflavin)
b) False. Complex I contains FMN
c) True. Complex II contains covalently bound FAD
d) False. Complete reduction of FAD requires 2 electrons
e) False. FAD is the prosthetic group of many oxidases present all over the cell
ATP synthase may catalyze ATP hydrolysis under uncoupled conditions. If the pH is decreased from 7 to 5 would you expect a change in the free energy of hydrolysis? Why?
ATP hydrolysis is accompanied by formation of a scalar H+ among the products:
ATP4- + H2O → ADP3- + Pi2- + H+
If we decrease the pH, we increase the [H+] in the medium, therefore we increase the concentration of products: the free energy of hydrolysis of ATP will therefore be less negative.
Isolated mitochondria are set to respire in an oxygen electrode with pyruvate + malate (5mM each). After addition of 10 µmol of ADP, the respiration rate remains in State 3 until 8 µg of oxygen are consumed, at a rate of 0.7 µg of oxygen per minute per mg protein. The rate of oxygen consumption in State 4 is 0.3 µg atoms per min per mg.
What is the RCI?
What is the P/O ratio?
What can you comment on the state of the mitochondrial preparation?
RCI = v state 3 / v state 4 = 0.7 / 0.3 = 2.3
P/O ratio (8 µg of oxygen are consumed to phosphorylate 10 µmol of ADP to ATP)
→ ATP/O ratio = 10/8 = 1.25 (State 3 remains until all ADP is phosphorylated to ATP)
P/O is a low value (theoretical with NAD-linked substrates is 2.5). Since also the RCI is low (good values are above 8) we may expect that the membrane is damaged and partly leaky to protons (high state 4 rate).
The protons translocated by Complex III originate
a) Only from ubiquinol
b) Only from H2O
c) From both ubiquinol and H2O
d) From none of them
c) From both ubiquinol and H2O
Analyse the mechanism of the Q-cycle:
2H+ from ubiquinol (QH2) oxidation are released in the intermembrane space
Another ubiquinol is oxidized as above with release of 2H+ in the intermembrane space
But at the same time 2H+ are taken from water in the matrix to resynthesize ubiquinol from the semiquinone
During State 3 respiration
a) The concentration of ATP increases
b) The membrane potential increases
c) The rate of oxygen uptake depends on ADP concentration
d) The amount of oxygen consumed does not depend on ADP concentration
e) The ADP concentration remains constant
a) The concentration of ATP increases
a) True. During State 3, ADP is converted into ATP
b) False. Membrane potential decreases because H+ re-enter via the ATP synthase
c) False. The rate stays constant until all ADP is converted to ATP. For the same reason d) and e) are also false
Respirasomes are supramolecular associations of Complexes I, III and IV. State the possible advantage of having a supercomplex association
The strict association between Complexes carrying out consecutive reactions has been proposed to allow direct transfer of electrons without a diffusion step in the regions of both CoQ and cytochrome c (channelling).
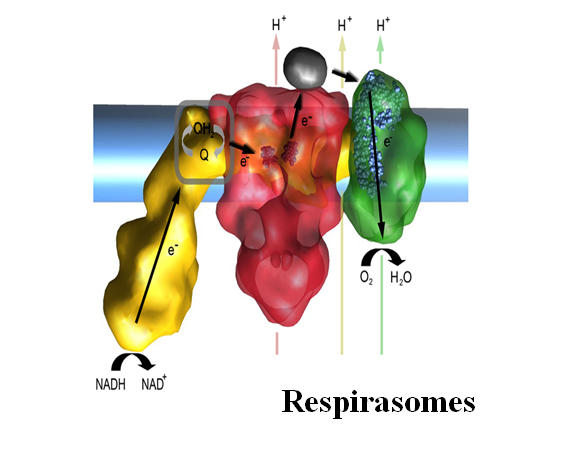
Approximately indicate the location of the following prosthetic groups:
FMN, heme b, heme a3, FeS clusters, CuA.
(Yellow = complex I; Red = Complex III. Green = Complex IV. Violet = cytochrome c)
FMN = hydrophilic part of Complex I
Heme b = hydrophobic part of Complex III
Heme a3 = hydrophobic part of Complex IV
FeS clusters = Complex I (7 clusters) and Complex III (1 cluster)
CuA = acceptor of electrons from reduced cytochrome c in Complex IV.
Only one answer is wrong. Mitochondrial cytochrome b
a) is a protein with two heme groups
b) is a trans-membrane protein
c) is contained in Complex III of the respiratory chain
d) is a carrier of hydrogen atoms
e) is encoded by mitochondrial DNA
d) is a carrier of hydrogen atoms
a) True. The two hemes are localized in the inner membrane thickness, near the two sides of the membrane
b) True. It has 8 transmembrane helices
c) True
d) False. It carries electrons (by oxido-reduction of Fe)
e) True. It is the only protein of Complex III that is encoded by mtDNA.
In the reaction represented below, what is the molecule indicated by X?
GSSG + X → 2 GSH + Y
a) Glucose-6-P
b) NADPH
c) ATP
d) H2O
e) None of the above
b) NADPH
The reaction represents the reduction of oxidized glutathione by glutathione reductase. The reductant is NADPH.
If we add dinitrophenol to mitochondria in State 4
a) The rate of oxygen consumption is unchanged
b) The rate of ATP synthesis decreases
c) There is inactivation of Krebs cycle
d) The inner mitochondrial membrane is less permeable to protons
b) The rate of ATP synthesis decreases
a) False. The rate increases
b) True. Uncouplers promote proton leak through the membrane, so they are not available for ATP synthesis
c) False. The increased oxidation of the chain maintains NAD in oxidized state and this activates Krebs cycle dehydrogenases
d) False. It is more permeable.
Only one answer is wrong. Coenzyme Q
a) May be reduced by 1 electron or 2 electrons
b) Is soluble in the membrane
c) When reduced may be re-oxidized by Complex III
d) Is reduced by Complex II
e) Is a vitamin, since it is not biosynthesized by humans
e) Is a vitamin, since it is not biosynthesized by humans
a) True. In the Q-cycle, one finds oxidized Q, semiquinone Q•- and ubiquinol QH2
b) True. Since it is lipid-soluble
c) True. It is re-oxidized by Complex III via the Q-cycle
d) True. Can also be reduced by some other dehydrogenases e.g. glycerol-P-dehydrogenase
e) False. It is biosynthesized by a complex pathway
Mitochondrial DNA
a) Is transmitted by maternal inheritance
b) Is contained in the intermembrane space
c) Encodes for 1 subunit of Complex II
d) Encodes for cytochrome c1
a) Is transmitted by maternal inheritance
a) True
b) False. It is in the matrix
c) False. No subunit of Complex II is encoded by mtDNA
d) False. In complex III, only cytochrome b is encoded by mtDNA
The “Rieske” Iron Sulfur Protein
a) Is encoded by mitochondrial DNA
b) Accepts one electron from ubiquinol
c) Is situated in the inner or negative side of the inner membrane
d) Is the reductant of cytochrome b
e) Has a redox potential more positive than that of cytochrome aa3
b) Accepts one electron from ubiquinol
a) False. It is encoded by nuclear DNA
b) True
c) False. It is situated close to the outer side of the inner membrane
d) False. It reduces cytochrome c1
e) False. It is more negative
Only one answer is false. Consider the addition of ADP to mitochondria respiring under State 4 conditions:
a) Added ADP is carried across the inner membrane by the Adenine Nucleotide Translocator
b) When ADP is exhausted, the rate decreases back to State 4
c) During State 3 respiration, ATP is synthesized
d) If we add oligomycin, ATP is not synthesized anymore, but the rate remains in State 3
d) If we add oligomycin, ATP is not synthesized anymore, but the rate remains in State 3
a) True. It enters in exchange with newly synthetized ATP
b) True. Because H+ do not re-enter any more through the ATP synthase
c) True. Exploiting the energy of H+ translocated by respiration
d) False. The rate decreases to State 4 or lower
If we inhibit with rotenone the respiration of mitochondria oxidizing glutamate, we expect
a) Decreased formation of α-ketoglutarate
b) Decreased ratio NADH/NAD+
c) Respiration is restored by an uncoupler
d) Coenzyme Q becomes more reduced
a) Decreased formation of α-ketoglutarate
a) True. If Complex I is inhibited, uphill reactions will also be inhibited; the rate of glutamate dehydrogenase decreases, so there is less product α-ketoglutarate
b) False. [NADH] increases
c) False. If the respiratory chain is inhibited, uncouplers cannot restore activity
d) False. Inhibition of Complex I will make CoQ more oxidized, since it is localized downhill of Complex I.
Oxygen is an excellent electron acceptor for the mitochondrial respiratory chain and supports high ATP yield because
a) It has a high value of E°’
b) It is very soluble in water
c) It has a very high chemical reactivity
d) It is reduced to hydrogen peroxide
a) It has a high value of E°’
a) True
b) False. Oxygen is more soluble in membranes than in water
c) False. It has a low chemical reactivity
d) False. It is reduced to water.
Submitochondrial particles obtained by ultrasonic irradiation are inside out with respect to mitochondria. During irradiation, the outer membrane and the matrix are lost. In the following list, indicate which items are exposed to the external medium, which are not accessible, and which are not present any more.
Binding site of glycerol phosphate in glycerol P dehydrogenase
F1 ATPase
Binding site of ETF
Pyruvate dehydrogenase
Cytochrome c
Mitochondrial DNA
Binding site of NADH in Complex I
Porin channel
Rieske iron-sulphur protein
FMN
pyruvate carboxylase
succinate dehydrogenase
Consider the topography of the mitochondrial membrane and that the vesicles have an opposite orientation. Thus:
Not accesible
Exposed
Accessible
Not present
Not accessible
Not present
Accessible
Not present (it’s in outer membrane)
Not accessible
Accessible
Not present
Accessible
The number of H+ flowing through F0 to energize rotation depends on the number of c monomers in the c-oligomer. One complete rotation generates 3 ATP. Depending on the species, the number of c monomers varies from 8 to 15.
Calculate the H+/ATP ratio for 8, 10, 12 and 15 monomers.
Calculate the P/O ratio with NADH and succinate oxidation in each case.
(Remember that 1 additional H+ for each ATP synthetized is required for translocation of ADP, ATP and Pi through the ANT and the Pi carrier.)
The movement of each subunit of the c oligomer allows translocation of 1H+; therefore the ratio H+/ATP = number of c monomers/3
2.67, 3.33, 4, 5 (e.g. 8/3)
P/O ratio = [H+ translocated during NADH oxidation (10) and succinate oxidation (6)] ÷ (number of H+ required to make ATP + 1H+ required for ANT and Pi carrier)
With NADH: 2.7, 2.3, 2.0, 1.7 (e.g. 10/3.7)
With succinate: 1.6, 1.4, 1.1, 1.0 (e.g. 6/3.7)
To isolated mitochondria in buffer in an oxygen electrode chamber, successive additions give rise to the following rates of oxygen consumption (relative values):
1
5
1
0.5
8
0.1
0
Combine each value with the following additions or conditions:
ADP, ADP depletion, FCCP, KCN, oligomycin, oxygen depletion, substrate + Pi
1 = substrate + Pi
5 = Addition of ADP triggers State 3
1 = ADP depletion brings back rate to State 4
0.5 = oligomycin (respiratory chain not inhibited → rate increases in next step)
8 = FCCP uncoupler inhibits oligomycin
0.1 = KCN inhibits respiration almost completely
0 = Oxygen depletion
How many moles of ferrous cytochrome c are required to reduce one mole of oxygen?
a) 1
b) 2
c) 4
d) 6
c) 4
O2 + 4e- + 4H+ → 2H2O
We need 4 electrons, therefore 4 moles of reduced cytochrome c.