Final Exam CH 101
1/144
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
145 Terms
Covalent Bond
Atoms of similar electronegativity (generally 2 nonmetals) share electrons
Ionic Bonds
transfer of electrons occurs due to atoms with a very large difference in electronegativity (metal and nonmetal)
Metal
Low ionization, low electronegativity (cations)
Non-metal
High ionization, high electronegativity (anions)
Ionization Energy
minimum energy needed to completely remove an electron from atom
Electronegativity
tendency to attract electrons
Naming Binary Ionic Compounds
name of cation followed by nonmetal anion with -ide ending
Transition Metals
+1 +2 or +3 charge (indicated by roman numeral)
Polar Covalent Bond
Difference in electronegativity is small (2 nonmetals)
Nonpolar Covalent Bond
Difference in electronegativity is ~ 0 (2 nonmetals or between C and H)
Bond dipole
unequal sharing of electrons that can exist in a covalent bond
Octet Rule
Nonmetals achieve closed valence shells of 8 electrons by sharing electrons
Steps for Drawing Lewis Structures
1) count # of valence electrons
2) if 2 atoms are present, draw a single bond (2 e-) between each atom (if 3 or more atoms are present, place the least electronegative, nonH atom in the center, then draw a single bond to connect the central atom to the surrounding atoms)
3) place all remaining valence e- on atoms as lone pairs
4) give octet to every atom that requires it
Bond Energy
Energy input required to break a bond
Bond length
distance between the nuclei of 2 atoms engaged in a bond
Formal Charge Formula
FC (atom) = # VE - Non Bounded - ½ B e-
Formal Charge definition
electron counting method for covalent bonds (assuming e- are shared equally) & hypothetical charge assigned to an atom as a tool to help evaluate molecular stability and predict reactivity
Resonance structure
alternate lewis structure depiction of the same molecule where atom connectivity remains the same, and the number of electrons remains the same… but the electrons are simply distributed around differently
Resonance Structure Hierarchy Rule 1
each atom that requires an octet has an octet
Resonance Structure Hierarchy Rule 2
Total number of atoms with nonzero formal charges is minimized
Resonance Structure Hierarchy Rule 3
If a formal charge has to exist (and it can be placed on different atoms), place the formal charge on the most appropriate atom (taking into consideration EN)
Convert the name “calcium chloride” into a chemical formula.
CaCl₂
Convert the formula Na₂SO₄ into a name.
Sodium sulfate
What is a polyatomic ion?
A covalently bonded group of atoms that carries a charge (e.g., NO₃⁻, SO₄²⁻).
What is a bond dipole?
An unequal sharing of electrons in a covalent bond, shown with a dipole arrow toward the more electronegative atom.
What are the octet rule exceptions?
Exceptions: H (2 e⁻), Group 3A (6 e⁻), expanded octets (row 3+).
Define bond order
Bond order = # of shared bonds
What is the relationship between bond order, length, and strength?
Higher bond order → shorter and stronger bonds. Lower bond order → longer and weaker bonds.
What is hybridization?
The mixing of atomic orbitals to form hybrid orbitals for bonding.
What is the ideal gas law?
PV = nRT.
Sigma bond
covalent bond formed by direct orbital overlap along the axis connecting the two nuclei
Pi bond
covalent bond formed by side to side overlap of p orbitals above and below the internuclear axis
Electron Domain
Area where electrons are likely to be found (lone pairs or bonds) around central atom
Electron domain geometry
3D arrangement of electrons around a central atom
Molecular geometry
3D arrangement of atoms in a molecule
Hybridized orbital
orbital formed by combination of atomic orbitals
bond angle and electron domain geometry with two electron domains?
180 degrees and lienear
bond angle and electron domain geometry with three electron domains?
120 degrees and trigonal planar
molecular geometry for four electron domains with two being lone pairs?
bent (109.5)
molecular geometry for four electron domains with one being a lone pair?
trigonal pyramidal (109.5)
molecular geometry for three electron domains with one being a lone pair?
bent (bond 120)
What is bond angle determined by?
The bond angle of a molecule is determined by the electron domain geometry, which is based on the total number of electron domains around the central atom, but is then influenced and distorted by the presence of lone pairs, leading to the specific molecular geometry.
Count σ bonds + lone pairs on the atom = number of hybrid orbitals.
4 → sp³
3 → sp²
2 → sp
hybridized orbitals make what type of bonds
make sigma bonds or hold lone pairs
un-hybridized p orbitals make what type of bonds
make pi bonds or remain empty
Molecular orbital
def: model used to describe the electronic structure of molecules using quantum theory. importance: detailed information regarding the electromagnetic properties of molecules
HOMO
highest occupied molecular orbital
LUMO
lowest unoccupied molecular orbital
antibonding molecular orbital
higher energy molecular orbital * (ant on E)
bonding molecular orbital
lower energy molecular orbital
paramagnetic
molecules with unpaired electrons, attracted to an external magnetic field
diamagnetic
molecules with all electrons paired, not attracted to an external magnetic field
difference of importance of hybridization and MO
hybridization gives the geometry of a molecule and its bonds, MO gives electromagnetic properties of molecules
difference in definition of hybridization and MO
hybrid- model used to describe the orbital structure of atoms engaged in bonding, MO model used to describe electronic structure of molecules using quantum theory
Ideal Gas Law
Pv = nRT
Partial Pressure
pressure a single gas in a mixture exerts if it alone occupied the volume
Intermolecular Forces
Attractive interactions that keep molecules together
Intramolecular Forces
Bonds within a molecule holding atoms together
London Dispersion forces
Temporary induced dipole that results from molecular motion (greater atom/molecule, greater force)- weakest Intermolecular (REQURIEMENTS- all molecules and atoms)
Dipole-dipole interactions
When molecular dipoles from two molecules are attracted to each other , second weakest intermolecular force (REQUIREMENTS: two polar molecules)
Hydrogen Bonding
When a partial positive hydrogen atom on one molecule bonds with a partial negative N O or F atom on a second molecule, strongest intermolecular force (REQUIREMENTS: partial (+) H and partial (-) N, O, or F) (NO CH)
Greater Strength of Intermolecular forces does what to boiling and melting points?
Greater Boiling and Melting Points
Molecular Dipole
the uneven distribution of charge within a molecule, resulting in a partially positive end and a partially negative end, which gives the molecule a net polarity and a dipole moment
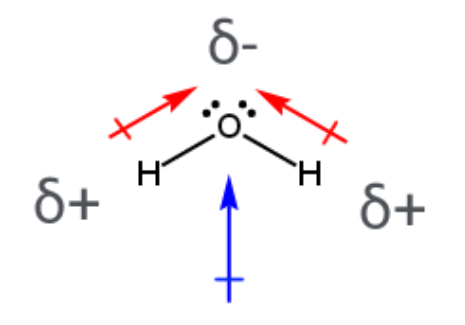
Solution
homogenous (uniform throughout) mixture containing solvent and solute
Molar Concentration
moles solute/ liters solution
Density
mass per unit volume of a substance (often g / mL)
Solution
Homogeneous mix of 2+ substances where one substance (solute) is dissolved in another (solvent)
Solute
Typically present in smaller amount- the substance that is dissolved into the solvent
Solvent
Typically present in larger amount- the substance that dissolves the solute
Solubility
The maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature to form a saturated solution.
Insoluble
Describes a substance (solute) that will not dissolve in a particular solvent.
Miscible
MIXABLE Describes two liquids that are completely soluble in each other in all proportions, forming a homogeneous solution (e.g., ethanol and water). (example of NON miscible is oil and water)
Detergent
a amphipathic (hydrophilic and hyrophobic parts) molecule made of a nonpolar hydrophobic tail and a polar hydrophilic head
Micelle
hydrophobic tails point inward and the hydrophilic heads point outward. (A spherical structure formed by the aggregation of detergent molecules in an aqueous solution)
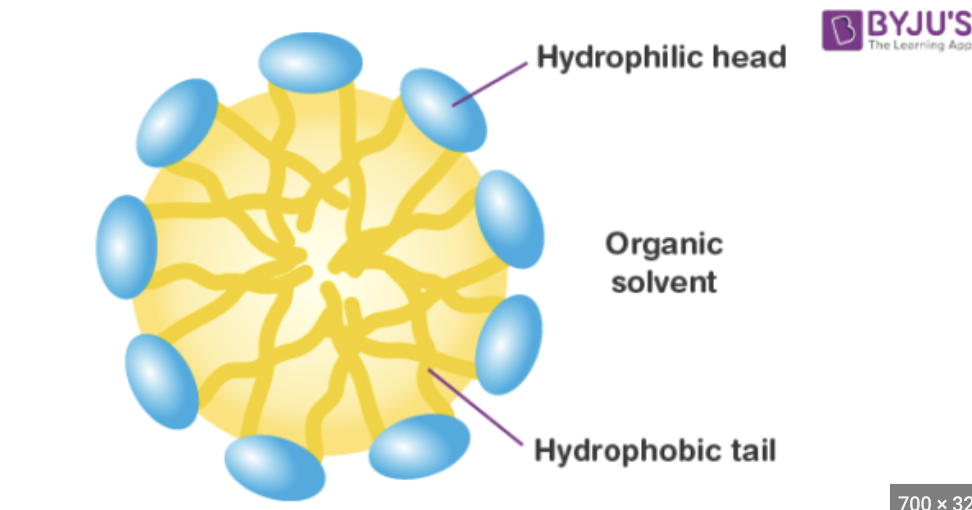
Function of a Micelle
It dissolves nonpolar (hydrophobic) substances like grease and oil by trapping them in its hydrophobic core, allowing the entire structure to be washed away by water.
Vapor Pressure
The pressure exerted by the vapor of a liquid in a closed container at a given temperature, representing the liquid's tendency to evaporate.
Boiling Point
The temperature at which the vapor pressure of a liquid equals the surrounding atmospheric pressure, causing the liquid to turn into a vapor.
Melting Point
Temperature at which a solid becomes a liquid at atmospheric pressure
Atmospheric Pressure vs. Vapor Pressure
When a liquid's vapor pressure is less than the atmospheric pressure, it remains a liquid. Evaporation occurs at the surface.
Atmospheric Pressure vs. Boiling Point
A liquid boils when its vapor pressure equals the atmospheric pressure. Lowering the atmospheric pressure (e.g., at high altitude) lowers the boiling point.
Thermodynamics
Are reactants or products energetically favored?
study of energy and its transformations- interested in starting and ending points in E
Kinetics
How fast will the reaction happen and by what mechanism?
study of the process by which reactants form products • Interested in rates and mechanism (how fast and by which route)
1st Law of Thermodynamics
Energy is neither created nor destroyed…but energy can be converted from one form to another
∆E universe = 0
∆E universe = ∆E surroundings + ∆E system
Endothermic
delta H > 0, heat energy absorbed by system causing surroundings to cool, enthalpically UNfavorable (ex: ice pack)
(within thermo and enthalpy H)
Exothermic
delta H < 0, heat energy exits system causing surroundings to warm, enthalpically FAVorable(ex: hot hands)
(within thermo and enthalpy H)
Enthalpy
heat content of reaction, (endo or exothermic)
Entropy
amount of disorder, related to the # of ways energy can be distributed in a system
Bond Dissociation Energy
the energy input required to break 1 mole of a bond in the gas phase
Weak bonds
more reactive; require less energy to break
Strong bonds
more stable; require more energy to break
∆ H formula relating to bonds
∆H = sum of Bond Dissociation Energy of bonds broken - sum of Bond Dissociation Energy bonds formed
Gibbs Free Energy Equation
∆G = ∆H -T∆S
T= Temperature (K)
Spontaneous
∆G<0, products lower in energy than reactants, reaction proceeds
Non-spontaneous
∆G>0, products higher in energy than reactants, reaction does NOT (non) proceed
Entropically Favorable
∆S>0 (POSITIVE) increase in disorder, nature likes disorder
Entropically Unfavorable
∆S<0 (NEGATIVE) decrease in disorder, nature likes disorder
Larger Ea means what for the speed of the reaction
Slower
Smaller Ea means what for the speed of the reaction
Faster
3 ways to speed up a reaction
Increase temperature
Increase concentration of reactants
Use a catalyst
Equilibrium
Point at which the forward rate = reverse rate and concentrations of reactants and products remains constant