bonding, structure, properties and energy changes
1/22
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
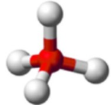
shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Tetrahedral, 109.5, 1 central atom, 4 regions of electron density, 4 bonds
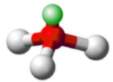
shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Trigonal Pyramidal, 109.5, 1 central atom, 4 regions of electron density, 3 bonds, 1 lone pair
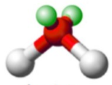
shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Bent (4), 109.5, 1 central atom, 4 regions of electron density, 2 bonds, 2 lone pairs
![<p>[only 1 green dot] shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)</p>](https://knowt-user-attachments.s3.amazonaws.com/4e5aa473-5c43-433b-a48a-1b98cdcb8342.png)
[only 1 green dot] shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Bent (3), 120, 1 central atom, 3 regions of electron density, 2 bonds, 1 lone pair

shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Linear, 180, 1 central atom, 2 regions of electron density, 2 bonds
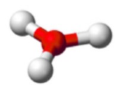
shape name, bond angle, no. of central atoms (e.g. 1,2,3), no. of regions (include full name), no. of bonds (no. of lone pairs if applicable)
Trigonal Planar, 120, 1 central atom, 3 regions of electron density, 3 bonds
Tetrahedral
109.5, 1 central atom, 4 regions of electron density, 4 bonds
Trigonal Pyramidal
109.5, 1 central atom, 4 regions of electron density, 3 bonds, 1 lone pair
Bent (4)
109.5, 1 central atom, 4 regions of electron density, 2 bonds, 2 lone pairs
Bent (3)
120, 1 central atom, 3 regions of electron density, 2 bonds, 1 lone pair
Linear
180, 1 central atom, 2 regions of electron density, 2 bonds
Trigonal Planar
120, 1 central atom, 3 regions of electron density, 3 bonds
Phrase to remember
Repel for maximum separation, resulting in minimum repulsion
Important water thing to remember
water (liquid) is a polar solvent - polar substances dissolve in it, water (H2O) is a polar molecule
Polar bonds are between…
atoms with different electronegativity
Non-polar bonds are between…
identically electronegative atoms in a symmetrical shape
Bond dipoles are… (δ)
representative of an unequal share of electrons/difference in electronegativity by the δ symbol, where δ+ represents the atom with the emptier shell (often the central atom)
Lithium chloride, LiCl (solid, particle, attractive forces, structure)
ionic, ions (a cation and an anion/metal and non-metal), strong ionic bonds, lattice and sea of delocalised valence electrons
Copper, Cu (solid, particle, attractive forces, structure)
metallic, metal atoms/metal cations and delocalised electrons, strong non-directional metallic bonds, lattice and sea
diamond, graphite, graphene, silicon dioxide (solid, particle, attractive forces)
3D covalent network, atoms, strong covalent bonds
water, H2O/oxygen gas, O2 (solid, particle, attractive forces, structure)
molecular, molecules (only one type or compounds of non-metals), weak intermolecular forces of attraction, lattice and sea of delocalised valence electrons
what is the ΔrH formula with energy unit (format ΔrH=___)
ΔrH=bonds broken-bonds formed, kJmol-1
Formula for energy (Energy=…, Δ)
Energy=ΔrHxn