Soil acidity/Alkalinity (Soil pH)
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Soil pH
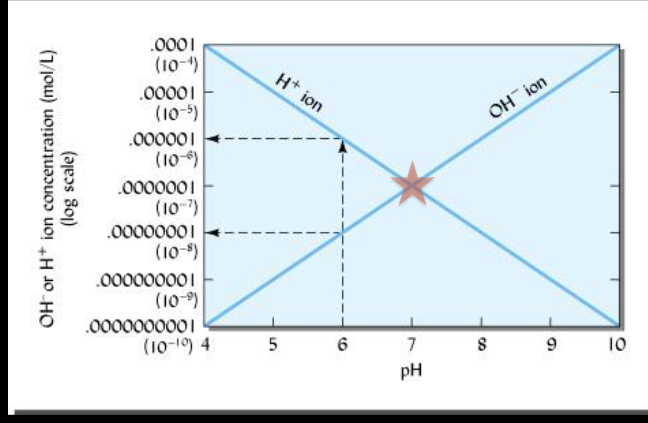
pH is the negative log of the H+ ion concentration.
Whether a soil is acid, neutral, or alkaline is determined by the
comparative concentrations of H+ and OH- ions.
soil pH
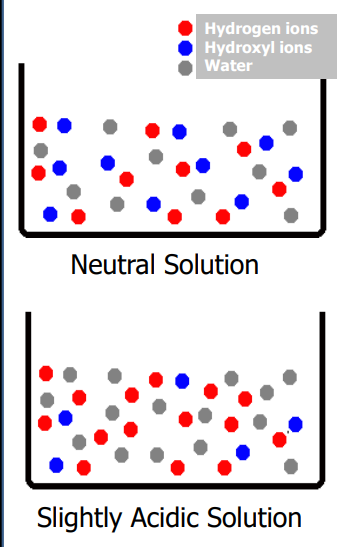
Acid solutions are
when pH is < 7.0
• Alkaline solutions are
when pH is > 7.0
• Neutral solutions are
when pH is 7
an acid is what
a proton donor - a chemical that increases the concentration of hydrogen ions; “produces” H+
a base is a proton what
proton acceptor - a chemical that reduces the concentration of hydrogen ions; “consumes” H+
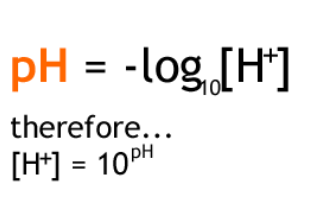
A basic review of pH
What is the [H+ ] of a soil solution with pH = 5.65?
[H+ ] = 1 x 10^-5.65
What is the difference in [H+ ] between pH 4.5 and pH 9.5?
Difference = 9.5 – 4.5 = pH 5 → [H+ ] = 1 x 10-5
why is Ph important
Greatly influences the root uptake availability of nutrients. • Influences the activity of microorganisms and type of vegetation that will grow in a particular soil. • Affects the mobility of many pollutants in soil – rate of biochemical breakdown, solubility adsorption to colliods
acidity and alkalinity
• Balance between hydrogen ions (H+ ) and hydroxyl ions (OH- ). • 2 processes promote soil acidification. 1. The production of H+ ions. 2. The washing away of nonacid cations (i.e., Na+ , K+ , Ca2+, Mg2+, etc.) • Soil acidity is closely related to the amount of annual precipitation.
Acidic soils (low pH)
• Humid climates – Intense weathering – Intense leaching • Basic cations (Ca+2, Na+ , Mg+2, K+ ) are replaced by H+ and Al(OH)2+
Basic soils (high pH)
• Arid climates – Lack of weathering – Lack of leaching • Carbonates accumulate at shallow depths
Acidifying processes that produce H+1. Carbonic and other organic acid
CO2 + H2O <-> H2CO3 - Metabolism of roots and microorganisms in the soil adds more CO2 . Other organic acids are created by biological activities in the soil
2. Accumulation of Organic Matter
• OM forms soluble complexes with nonacid nutrient cations (Ca2+ and Mg2+) facilitating their loss by leaching. • OM contains numerous acid functional groups from which H+ ions can dissociate.
3. Oxidation of Nitrogen (Nitrification)
NH4 + + 2O2 <-> H2O + H+ + H+ + NO3 - Oxidation reactions generally produce H+ ions as a product. The reaction of NH4 + with oxygen releases 2 H+ ions for each NH4 + ion oxidized.
4. Oxidation of Sulfur
FeS4 + 3 ½ O2 + H2 FeSO4 + 2H+ + SO4 - When sulfur is oxidized, the reaction yields sulfuric acid (H2 SO4 ). Responsible for large amounts of acidity in sulfur containing soils that are exposed to oxygen through drainage or excavation
5. Plant uptake of Cations
For every positive charge taken in as a cation, a root can maintain the necessary charge balance by taking up anions or by exuding a different cation. soil solution
Uptake of cations balanced by the release of H+ from the root – an acidifying effect.
Uptake of cations balanced by uptake of anions – no effect on pH.
no releasing any hydrogen ions
active acitidity
due to the H+ ion activity in the soil solution at any given time.
reserve acidity (on sites)
represented by the H+ and Al3+ that are easily exchanged by other cations
active measuring after 20 mins
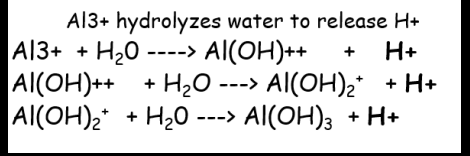
Role of Al in soil acidity
• Al3+ ions have a strong tendency to hydrolyze.
• Al3+ combines with OHleaving the H+ to lower the pH of the soil solution.
• This is why Al3+ and H+ are considered acid cations.
• A single Al3+ ion can release up to 3 H+ ions.
releasing hydrogen ions into the soil
slightly acid levels hydrogen ion aluminum ion levels
Human-influenced soil acidification
Natural processes of acidification are greatly (although inadvertently) accelerated by human activities.
1. Nitrogen amendments
2. Acid precipitation
3. Exposure of potential acid sulfate soils

Nitrogen Amendments and pH
Application of N fertilizers can decrease pH over time and space. A lot of agriculture taking place how deep it traveled through the soil profile. huge loss monitor weather. fertilizers may not be the best methods calls ask do we need to apply less.
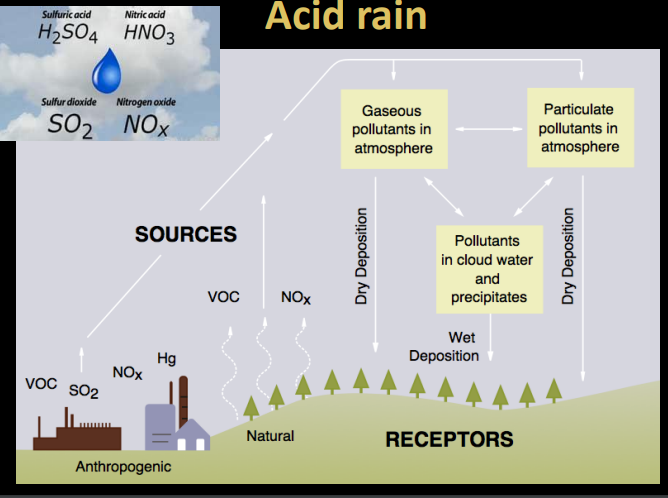
Acid rain and damages
acid rain very difficult to manage. released from industrial processes. under both wet and dry conditions. ghost forests completely dead

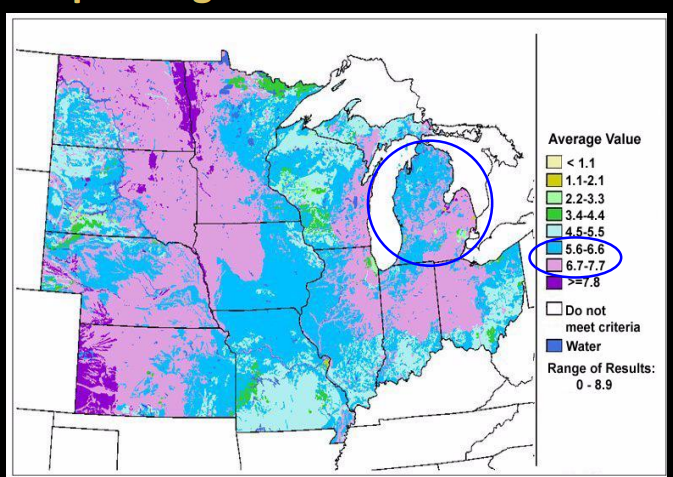
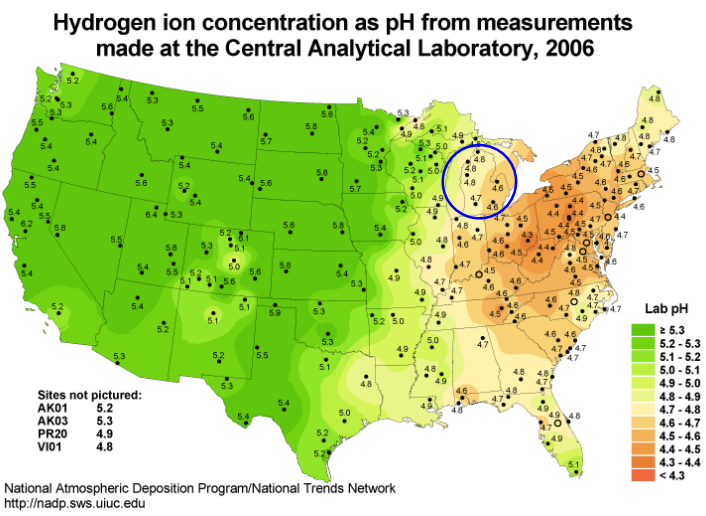
pH range of midwestern soils
In michigan we have a lot of industrial activity in past. in new hampshire hubbard brooke lots of ecological research in 60s 2 researchers saw water and soil pH came all the way from industrial midwest carried to the east coast. in these soils pH of 4.6 bad pH impact vegetation and organisms. solution moving forward prevention
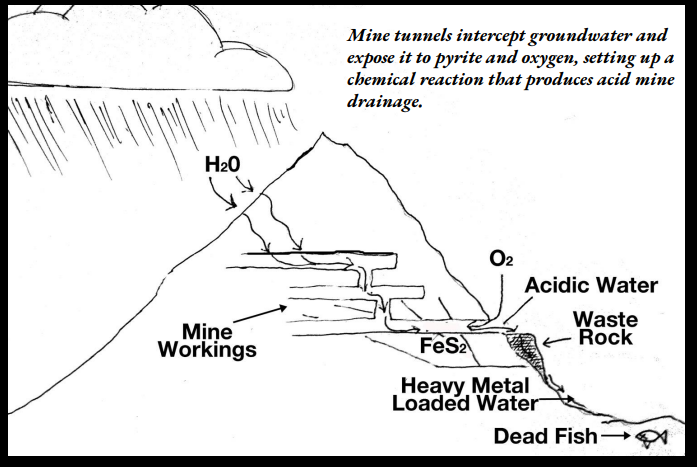
Acid Mine Drainage (AMD)
Pyrite is commonly present in coal and in the rock layers overlying the coal.
Pyrite will react with water and oxygen to form iron compounds and sulfuric acid.
FeS2+O2+H2O——>Fe(OH)3+H2HSO4
the products of AMD formation, acidity and iron, can devastate water and soil resources
acid soils

Alkalizing processes that consume H+ or produce OH-.
Weathering of non-acid cations from minerals Ca-silicate + 2H+ <-> H4 SiO4 + Ca2+
• Non-acid cations (Ca2+, Mg2+, K+ , Na+ ) are released by weathering may become exchangeable cations on soil colloids.
• H+ may replace these on the exchange sites and the now displaced cations are subject to leaching loss.
cations in solution prone to leaching coarser textures increase leaching
Alkalizing processes that consume H+ or produce OH-.
2. Accumulation of non-acid cations
In arid climates, where precip < ET, cations released by mineral weathering accumulate.
• The cations on the exchange sites are nonhydrolyzing and do not produce acid (H+ ) upon reaction with water as do the acid cations (Al3 + and Fe3 + ).
Alkalizing processes that consume H+ or produce OH-.
Production of Base-Producing Anions
The basic, OH- generating anions are mainly carbonate (CO3 2- ) and bicarbonate (HCO3 - ) and originate from calcite or from the dissociation of carbonic acid.
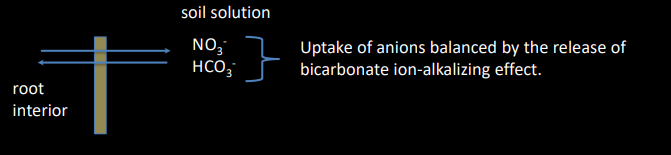
Alkalizing processes that consume H+ or produce OH-
Excess anion uptake by roots
When plant uptake of an anion exceeds the uptake of associated cations, the roots exude the bicarbonate anions to maintain the charge balance.

hydrageas
blue colors acidic
purple medium
pink high pH
Why could there be different colored hydrangeas adjacent to each other or on the same plant?
Spatial variability
proximity to plant roots, concentration of fertilizers or ash from a forest fire, erosion, drainage …
• With soil depth –
in many instances, soil pH in the upper horizons is lower than in the deeper horizons – unless there has been human application of liming materials.
Biological effects of soil pH
The pH of soil is a critical environmental factor for the growth of all organisms that live in the soil including plants, animals, and microbes. Brady & Weil
Relationships between pH and the availability of plant nutrients
In acid soils, Ca, Mg, K, P, N, S, Mo, and B availability is low.
• In alkaline soils, Fe, Mn, Zn, Cu, Al, and Co are so low that plant growth is constrained.
• In general, pH 5.5 – 7.0 may provide the most satisfactory plant nutrient levels.
Thicker the band more nutrient availability for optimal growth.
Aluminum toxicity
Most common and severe problem associated with strongly acid soils (low pH). ph 5 or below
• Rarely a problem above pH 5.2 b/c little Al exists in the soil solution or on exchange sites.
• There is an exponential increase in Al3+ concentration as the pH drops from 5 to 4.

Al toxicity in plants
Enters the root and damages membranes and
restricts cell wall expansion so roots cannot grow
properly.
• Stunted root system with short, stubby roots that
show little branching or growth of laterals.
Al toxicity in plants pt 2
Root tips and lateral roots often turn brown.
• Leaves may show chlorosis (yellowing) and often
show symptoms of drought stress and P deficiency.
Manganese toxicity in plants
Not as widespread as Al toxicity but is a
serious concern for plants growing in acid soils
derived from Mn-rich parent materials.
• Toxic when taken up in excessive amounts.
• Symptoms include crinkling or cupping of
leaves and interveinal patches of chlorotic
tissues.
Microbial effects
• Fungi are particularly versatile and thrive over a
wide range of soil pH. limitations still involved
• Bacteria are strong competitors and dominate at
moderate to high pH while fungi predominate at
low pH.
• Manipulation of soil pH can be used to control
certain soilborne plant diseases and
decomposition processes. under different moisture conditions
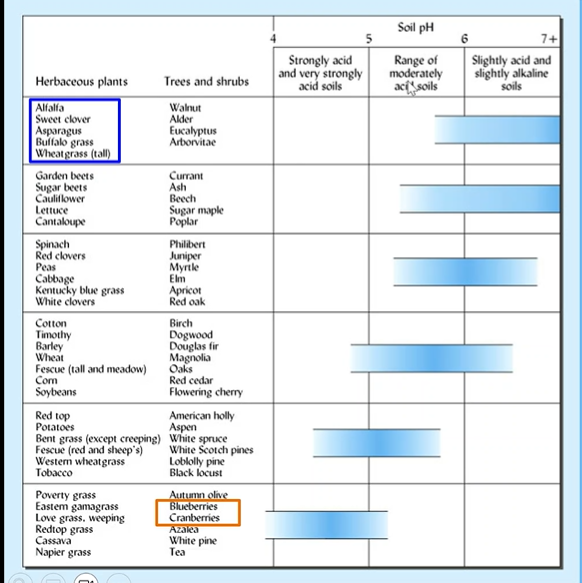
Optimal soil pH for plant growth
Most cultivated crops grow well on soils that are just slightly acid to near neutral
5.5 to 7
blueberries and cranberries like acidic soil
Salt-Affected Soils
Salt-Affected Soils
• Cover 320 million ha of land throughout the
world – largely in Australia, Africa, Latin
America, SW United States, and the Near and
Middle East.
Saline soils
Saline-Sodic soils
Sodic soils
Electrical conductivity
Indirect measurement of salt content.
• Conductivity increases as more salt is
dissolved in the soil water.
• Measure of soluble salts.
typical EC values dS/m
rainfall 0.030
sweage effluent 0.80-1.50
fresh streams 0.039-0.500
Ag upper limit 8.00
Brackish 1.50-15.00
Seawater 55.00 very salty why we float so well
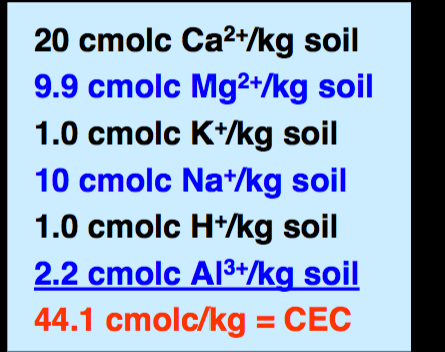
Exchangeable Sodium Percentage
• Characterizes the Na status of soils.
• Degree to which the exchange sites are
saturate with sodium.
• Synonymous with Na saturation.
Calculate the ESP%
divide 10 by 44.1 then multiply by 100 because we are only accounting for sodium in the soil
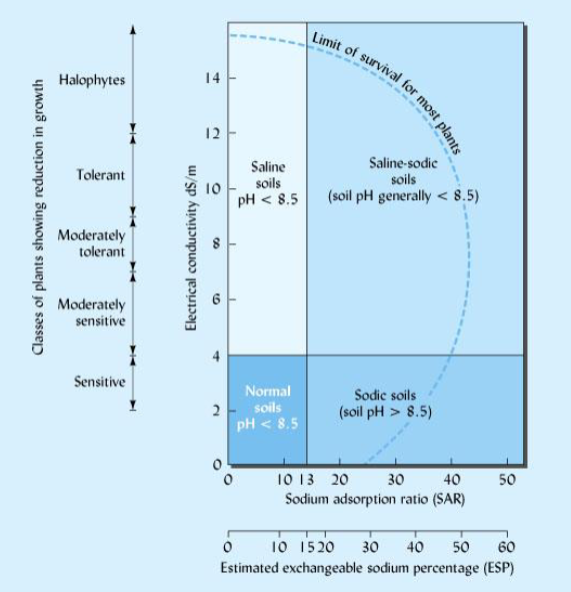
salt affected soils
saline
high EC
low ESP
pH typically < 8.5
-saline-sodic(great for plant growth)
high EC
high ESP
pH typically < 8.5
sodic
low EC
high ESP
pH > 8.5
sodic soils. 3 main problems of Na-induced dispersion
– Reduced infiltration
– Reduced hydraulic conductivity
– Surface crusting
clays are attracted to each other edge to edge but if you have a enough sodium they will repell each other
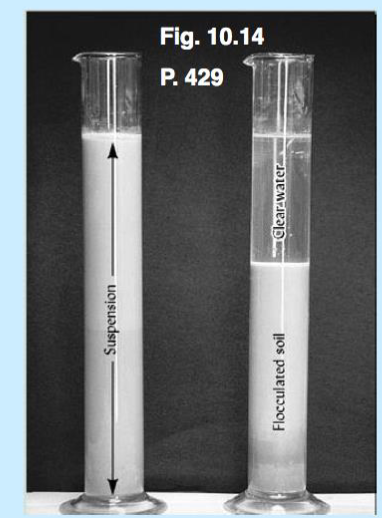
dispersion and flocculation
sodic soil salty!! if you do effervescent test and it doesnt bubble it has sodium
