(pt 2) exam #4 - immunohematology (cls 544)
1/73
Earn XP
Description and Tags
component preparation and transfusion therapy
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
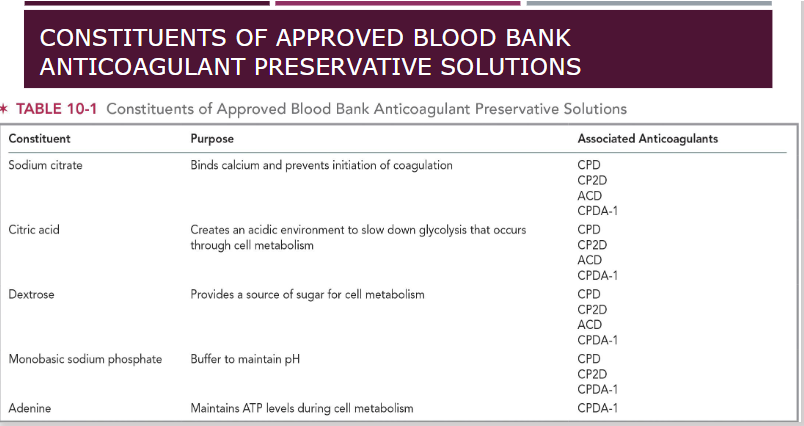
purpose of anticoagulant preservative solutions
Prevent activation of clotting factors
Citrate-based, bind calcium and blocks the coagulation cascade
Maintain blood in liquid transfusion
Extends the shelf-life of blood components
***ACD, CPD, CP2D have a shelf life of 21 days
***CPDA-1 shelf life 35 days
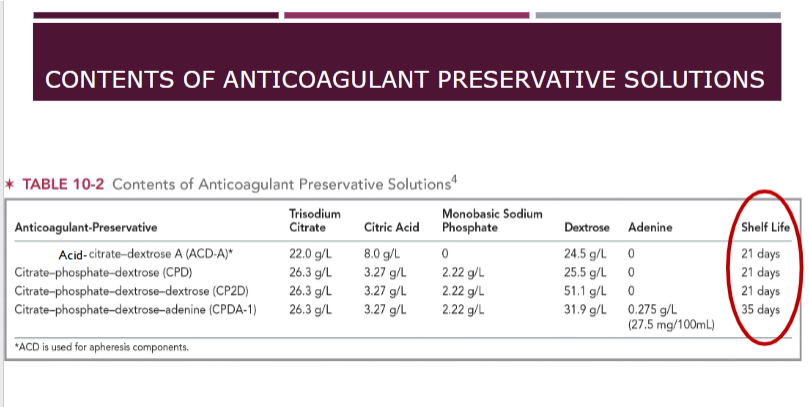
production of blood components from whole blood
Whole blood collected from donor into plastic collection bag containing anticoagulant preservative
Satellite bags are storage containers for blood components prepared from donation
e.g. plasma, platelets
The closed system ensures no foreign contaminants from the outside environment can enter system
(component preparation) whole blood
Comprised of all plasma and cellular components of blood
Not typically used for transfusion
Separating whole blood into components allows multiple patients to benefit from single blood donation
WB volume: ~450-500 mL
Hematocrit ~38%
(component preparation) packed RBCs
Prepared by centrifuging whole blood
During centrifugation, the RBCs concentrate at the bottom of the container, followed by white blood cells and platelets
Top portion of centrifuged collection bag contains liquid plasma portion of whole blood
(component preparation) processing of packed RBCs
After centrifugation, blood unit plasma expressor
RBCs and plasma bags separated using tubing sealer
Packed RBCs component:
If additive solutions (AS) used, Hct ~55 to 65%
If AS not used, Hct 65-80%
50 to 80 grams of hemoglobin, final volume ~160 to 275 mL
ASI, AS3, AS5, AS7--42 days of storage
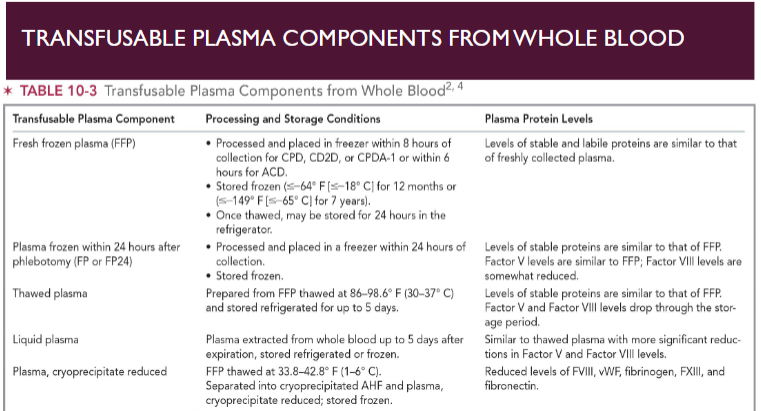
(component preparation) plasma
Stored frozen to preserve activity of labile plasma proteins
e.g. Factors V and VIII
Frozen plasma thawed prior to transfusion
Stored in thawed state for short periods of time
1 unit of each coagulation factor in every 1 mL of FFP
WB-derived plasma volume range from 200-375 mL
(component prep) solvent/detergent & recovered plasma
Solvent/detergent plasma
Used to inactivate lipid-enveloped viruses such as HIV, HBV, HCV, and CMV
recovered plasma
Proteins separated out for patient use
e.g. albumin, specific coagulation factors
(component preparation) cryoprecipitated antihemophilic factor (AHF)
Frozen FFP thawed slowly at 1-6 C; insoluble proteins precipitate out of the plasma in the cold
Fibrinogen, Factor VIII, Factor XIII, von Willebrand factor (vWF) and fibronectin
15 mL of residual plasma is left with the insoluble proteins
Each unit must contain a minimum of 80 IU of Factor VIII and 150 mg of fibrinogen
remaining plasma (minus the cryo) is called cryoprecipitate reduced plasma
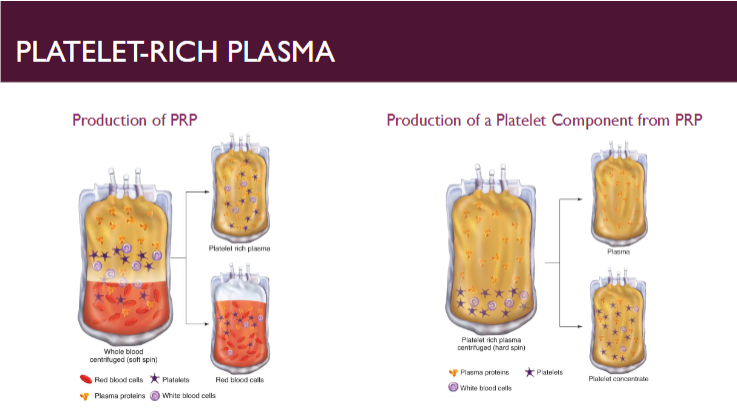
(component preparation) platelets (PRP method)
Donations maintained at RT during all stages of collection, storage, and production
Platelet-rich plasma (PRP) method
Produces a single platelet component from single WB donation, random-donor platelets (RDPs)
Contains a minimum of 5.5 x 1010 platelets
Total volume 40-70 mL
Residual RBCs cannot exceed 1.0 x 109 RBCs per component
One therapeutic dose for adult equivalent to 4-6 PRP platelets, each prepared from one WB donation
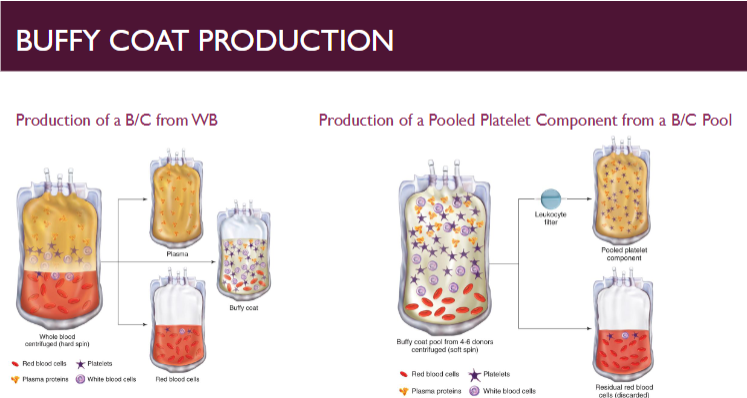
buffy coat (B/C) method for platelet preparation
produces pool of plts from 4-6 whole blood donations
Whole blood subjected to hard spin
Second stage starts with pooling buffy coats with one plasma component or bag of platelet additive solution
Most common in Europe and Canada
One pooled B/C platelet is generally considered a normal adult dose
(component preparation) granulocytes
White blood cells are not often prepared from whole blood
Apheresis collection technique results in higher number of granulocytes per collection
Buffy coat isolated by using hydroxyethyl starch (HES) to precipitate out the WBCs
1.0 x 10^10 granulocytes
Shelf life 24 h--transfused ASAP
Stored at room temperature
blood component labeling
ISBT 128 Labeling includes:
Donation ID number; blood group of donor
Product code and description
Expiration date & Special testing done
Label provides all information necessary to handle product appropriately
Circular of information
(storage of blood components) adverse effects of RBCs storage
Blood removed from body undergoes changes and can influence how components function
Apoptosis = programmed cell death
Hemolysis is the most obvious impact of storage on RBCs
Freed hemoglobin is released into the storage fluid
Metabolic activity and oxygen release reduce levels of ATP and 2,3-DPG
Many of these changes are reversible after transfusion
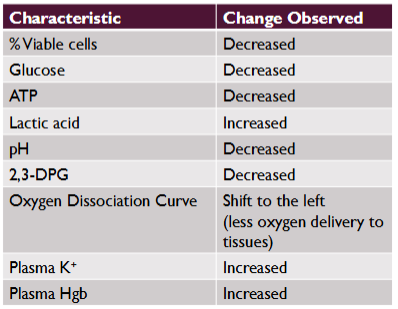
(storage of blood components) RBC viability
Loss of RBC viability has been correlated with the lesions of storage
Associated with various biochemical changes within the pRBC unit
Glucose, ATP, pH, and 2,3-DPG, # of viable cells are all decreased
Lactic acid, plasma K+, and plasma Hgb are all increased
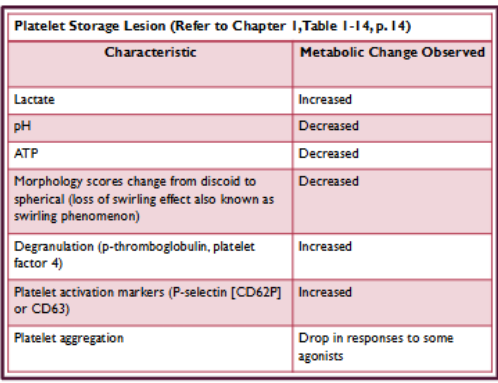
(storage of blood components) adverse effects of storage on platelets
Very fragile and sensitive
Adversely affected by temperature and forces applied during centrifugation
High levels of metabolic activity during storage
Prolonged storage eventually leads to cell death
platelet storage lesion (see pic)
Quality control measures
Platelet concentrations
Platelet concentrate volume
Platelet count
pH of the platelet unit
Residual leukocyte count (if leukoreduced)
Visual inspection--assess platelets swirl (observe for any platelet aggregates)
Loss of swirl indicates loss of membrane integrity during storage
adverse effects of storage (general)
Cellular blood components most susceptible to storage lesion
Transfused cells must remain intact and metabolically active
Plastics used in collection and storage containers affect cell function
Anticoagulant preservative solutions play significant role in reducing storage lesion
Storage conditions
Temperature and length of time of storage
Colder storage temperature = longer component may be stored
Providing continuous agitation to platelet components important!
Use of additive solutions prolongs storage time for cellular components
e.g. red blood cells
(storage of blood components) adverse effects on granulocytes, plasma, and bacteria considerations
Granulocytes
Fragile and deteriorate quickly after removal from body
Rapid deterioration = release of cytokines and intracellular enzymes = cell death of RBCs and platelets
Plasma protein
Most very stable; factors V and VIII breakdown when plasma is stored in the refrigerator or room temp
Bacteria
Risk for minute numbers of bacteria may enter the closed system
Bacterial metabolism can accelerate RBC, WBC, and platelet destruction
Can cause severe reactions in transfused patients
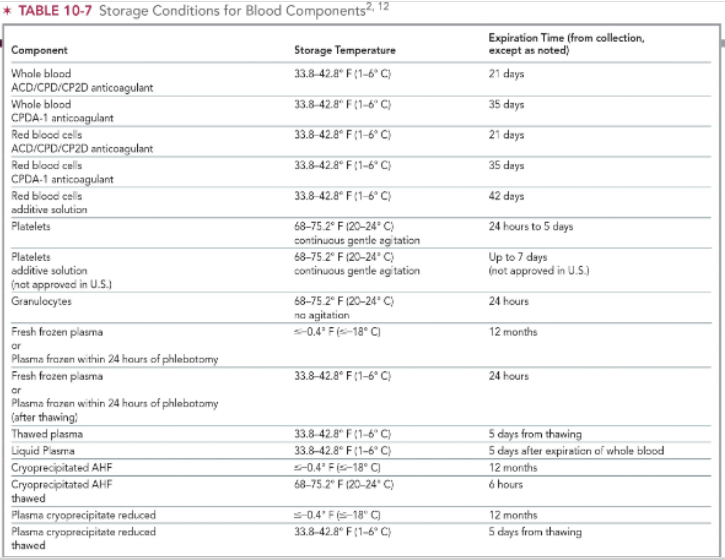
storage conditions for RBC products
whole blood/pRBC + ACD/CPD/CP2D = 1-6 C
expires 21 days from collection
whole blood/pRBC + CPDA-1 = 1-6 C
expires 35 days from collection
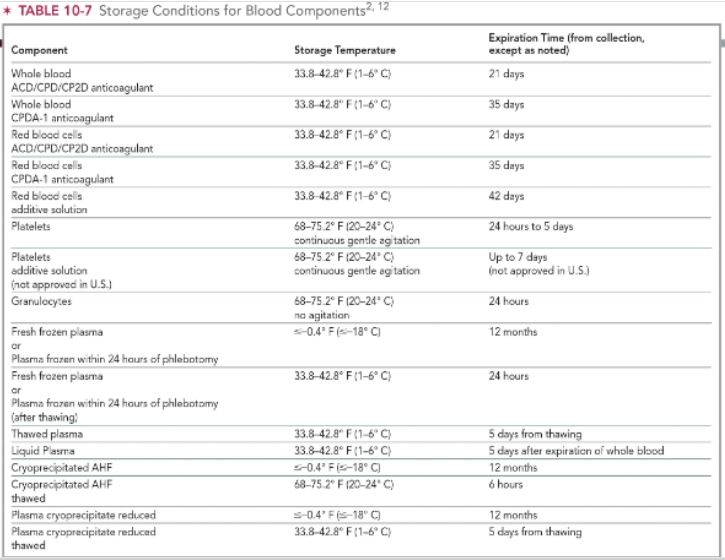
storage conditions for platelet products & granulocytes
both store at 20-24 C
platelets need continuous gentle agitation
expiration time:
up to 7 days for platelets
24 hrs for granulocytes
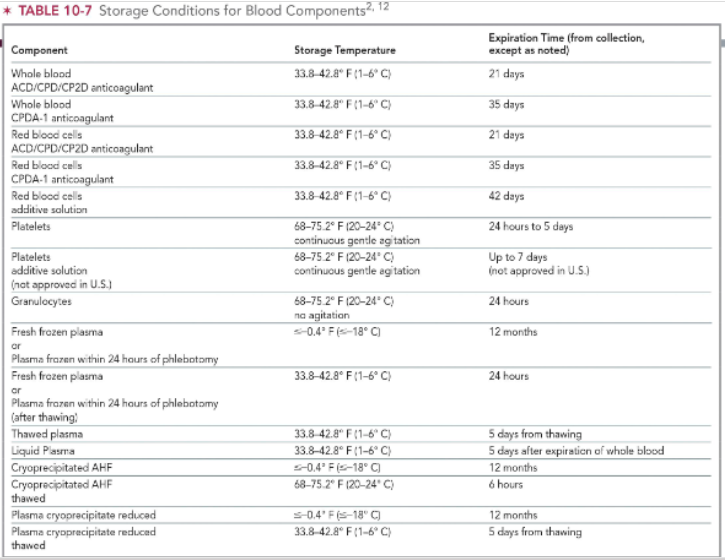
storage conditions for plasma products
FFP: store at < -18 C for up to 12 months
same for cryoprecipitated AHF & reduced cryoprecipitate
FFP after thawing: store at 1-6 C for up to 24 hours
thawed plasma: store at 1-6 C; expires 5 days from thawing
liquid plasma: store at 1-6 C; espires 5 days after expiration of WB
thawed cryoprecipitated AHF: 20-24 C for up to 6 hours
reduced plasma cryoprecipitate thawed: 1-6 C; expires 5 days from thawing
transportation of blood components
Blood collected in donor clinic must be transported to production laboratory for component prep
Blood separated into components, stored, and labeled upon review of serological/viral marker test results
Must have the ability to maintain temperature range for components being shipped (1 C - 10 C)
Phase-change materials are a compound that shift between solid and liquid state at specific temperatures
(blood component modifications) leukoreduction
Removes WBCs from whole blood or blood components & helps to prevent formation of antibodies to HLA antigens because WBCs carry HLA antigens
Reduces risk of adverse reactions in transfused patients & decreaes viral load of CMV, HTLV, & EBV
e.g., HLA, TRALI, organ rejection (TA-GVHD), plt refractoriness
Leukoreduction filter may be used
Leukocyte reduction filters remove 99% WBCs (<5×106/unit)
Acceptable number of remaining leukocytes = 5 x 10^6 per component
(blood component modifications) pathogen inactivation
Process that inactivates viruses and bacteria in prepared blood components
Solvent and detergent treatment: HIV, HBV, HCV, HTLV, EBV, CMV, HHV-6, HHV-8
Plasma treated using methylene blue, psoralen (platelets), or riboflavin
(blood component modifications) aliquoting & pooling
Aliquoting
To prepare product for low-volume transfusion, usually neonatal or pediatric patients
Can be done in a closed or open system
Pooling
Reduce number of bags accessed at time of transfusion
Commonly done with PRP platelets or cryo to create a single dose
(blood component modifications) volume reduction
Remove plasma or additive solution from components
Done via centrifugation
Reduces risk for circulatory overload and adverse reactions related to transfusion of certain plasma proteins
(blood component modifications) irradiation & washing & rejuvenation
Irradiation
Exposing blood components to gamma or x-ray radiation inactivates T lymphocytes
No adverse effect on platelet or red blood cell function
Increased potassium leakage into the storage fluid
Washing
removes plasma proteins that can cause severe reactions in certain patient populations
Rejuvenation
Rejuvenates and restores RBCs up to 3 days after expiration with FDA-approved solution
(blood component modifications) freezing, thawing, deglycerolizing RBCs
Freeze and store RBCs with rare phenotypes for long period of time (below -65 C, 10 years)
RBCs normally undergo cell lysis when frozen, then thawed
Glycerol (high-40%, low-20%) limits formation of ice crystals within RBCs, reducing damage to cell membrane
Deglycerolization
Washing thawed component with successive concentration changes of sterile saline solution, starting with hypertonic saline, 1-6 C, open system for 24 hours; closed-14 days
RBCs that are not properly leukoreduced can produce what adverse reactions?
Febrile, non-hemolytic reaction
Reaction to cytokines
Alloimmunization to HLA or granulocyte antigens
Transmission of CMV
plasma fractionation
Plasma not used for transfusion can be shipped to large manufacturing facilities
Solvent/detergent treatment
Heat or nanofiltration process
Facilities separate out specific proteins and package them to meet specific patient needs
Albumin (5% or 25%)
Immune globulins (e.g. RhIG, IVIG)
Coagulation proteins
FVIII, FIX, fibrinogen, anti-thrombin III, protein C
recombinant protein production
Produced by inserting genetic information that codes for specific human protein into DNA of plant or animal cells
Production does not rely on availability of donor plasma
Manufacturers use methods that use no human or animal derived proteins during production
Completely within manufacturer's control
Factor VII and Factor IX in use in recombinant form rather than plasma derived
blood banks without collection facilities
Blood banks that depend on an outside source for their blood supplies usually receive their products in component form
Platelet concentrates and cryoprecipitate may be received as individual units but are more easily administered if pooled before infusion
Individual units of platelets outdate in 5 days
Pooled platelets in open system are good for 4 hours
Proper timing for utilization is critical
Sterile connecting devices (STCDs) have also become commonplace in some blood banks
blood bank equipment for storage / processing of blood products
Refrigerators
Packed RBCs and whole blood—1-6 C
Storage requirement for transportation of RBC: 1-10 C
Freezers
Maintained at -18 C or lower for FFP, FFP24, and cryoprecipitate
Maintained at -65 C or lower for frozen RBCs (40% glycerol)
Platelet Rotators/Flatbed Agitators
Provide constant gentle agitation @ RT for storage of apheresis and random donor platelets
20-24 C
Automated Cell Washer
Used to prepare washed RBCs or to deglycerolize frozen RBCs
(blood bank instruments) irradiator
used to render donor T lymphocytes nonfunctional in whole blood, red blood cells, platelets, and granulocytes prior to transfusion
Prevents development of Transfusion Associated Graft-Versus Host Disease (TA-GVHD) by gamma irradiation of cellular blood products
Storage-life of red blood cells and whole blood reduced to 28 days or original outdate, whichever is sooner
Immunocompromised patients, fetus and neonates, transplant patients, or patients receiving transfusion from a relative (directed donation) are at increased risk of TA-GVHD
goals of tranfusion therapy
Common goals
Increase oxygen-carrying capacity
Restore hemostasis
Laboratory testing
CBC is the first laboratory test performed to assess need for transfusion
Common coagulation screening tests
PT, aPTT, INR, and fibrinogen
transfusion therapy (general)
Blood components are deemed as "drugs" by the FDA
RBCs are the best tolerated blood product for transfusion
Rejection of platelet components are relatively common for patients who have received multiple platelet transfusions
Appropriate transfusion therapy requires proper selection of blood products for treatment and optimization of blood product inventory
decision to transfuse blood into a patient is not made lightly
Severely anemic patients may require an infusion of pRBCs to increase O2-carrying capacity of the blood
Platelets given to patients with decreased platelet quantity or function
Plasma given to patients who require an infusion of coag factors
transfusion triggers for RBCs + expected response
trigger: hgb < 7 g/dL; higher if there are underlying cardiac/pulmonary disease
common adult dose: 1 unit
expected response: hgb increases by 1 g/dL per dose
transfusion triggers for FFP + expected response
trigger: aPTT/PTT increases greater than 1.5 upper limit or INR > 1.5
common adult dose: 2 whole blood derived units/1 apheresis unit
expected response: coag factors increase by 25%
transfusion triggers for platelets + expected response
trigger: platelet count <10 × 10³/uL
common adult dose: 1 apheresis unit or 4-6 pooled whole blood derived units
expected response: increase of 30-50 × 10³/uL 1 hr post transfusion
transfusion triggers for cryoprecipitate + expected response
trigger: fibrinogen <100 mg/dL
common adult dose: 10 units or 1 unit/10kg body weight
expected response: 14 units = increase fib by 50 mg/dL
transfusion triggers for granulocytes + expected response
trigger: documented sepsis unresponsive to antimicrobial therapy
common adult dose: 1 unit
expected response: no demonstrable rise in neutrophil count
(transfusion therapy) categories of blood products
primary function of the blood bank is to supply the safest and most suitable blood and blood products to patients as quickly as possible
Cellular components: whole blood, red blood cells (packed RBCs), platelets, and granulocytes
Plasma components
FFP, PF24, and thawed plasma
Cryoprecipitate
Platelet products
Hematopoietic Progenitor Cell (HPC) Products
Bone marrow, peripheral blood stem cells (PBSC), and cord blood
Plasma Fractionation Products
Albumin, immune globulins, and coagulation factor concentrates
(transfusion therapy) infusion rate
All blood components should be infused in LESS THAN 4 HOURS
Minimal risk of bacterial contamination
Refrigeration inhibits bacterial growth
Infusion rate for each patient document
First 10-15 min: infused slowly, vital signs
(transfusion therapy) infusion sets and filters
Set used dependent of the time limit or maximum number of units being infused
Standard set manufactured with flexible plastic tubing and an inline blood filter
Pore size: 150-260 um
Traps large cellular debris
(infusion sets and filters) microaggregate & leukocyte reduction filters
Microaggregate filters for RBC transfusions
Pore size: 20-40 um
Traps degenerating platelets, WBC fragments, and small strands of fibrin
Pore size slows down infusion rate
Leukocyte Reduction Filters
Reduce the number of WBCs in RBC (or PLT) components to less than 5 × 106 WBCs/unit
Filters are not interchangeable between blood components
Can occur pre-storage while components are being prepared or before transfusion at the bedside
(infusion considerations; transfusion therapy) needles, rapid infusers, blood warmers
Needles
Small bore sizes can cause hemolysis during rapid infusion of RBCs
18 gauge or larger needles used for transfusion
Smaller needles (23 gauge) used for pediatric patients
Use slow infusion rate for smaller needles and large bore needles for rapid infusion of RBCs
Rapid Infusers
Resemble pressure cuffs that surround entire bag of blood
Massive rapid infusion or infusion into central venous catheter presents a higher risk of hypothermia in recipients
Hypothermia can cause arrhythmia which can be fatal
Blood Warmers: prevent hypothermia and patients with cold agglutinin disease
(transfusion therapy) infusion pumps and infusion solutions
Infusion pumps
Mechanical pumps used to regulate blood flow into patients
Often used in neonates--volume shifts has a drastic impact
Infusion solutions
Normal saline of 0.9% sodium chloride (USP) may be added to most blood components to increase flow rate
Safe to add RBC units
ABO-compatible plasma, 5% albumin, and Plasma-Lyte
Medications, intravenous solutions, Ringer's lactate solution should not be added to blood components during transfusion
Some solutions not compatible with fractionated products
(transfusion therapy) whole blood
Comprised of RBCs, platelets, and plasma
Originally used for volume replacement (plasma)
Improve oxygen-carrying capacity (RBCs)
Volume replacement treated with crystalloid solutions or colloid solutions
Component therapy replaced use of whole blood
If whole blood used, must be ABO identical and crossmatched
Typically encountered with presurgical autologous donation
(transfusion therapy) RBC components
referred to as "packed cells" or "packed red blood cells"
Commonly used to treat anemia
Whole blood and pRBCs improve oxygen transport
Transfusion Triggers
Hemoglobin falls between 7–10 g/dL
Decision to transfuse should not be based only on laboratory findings
effects of transfusing packed RBCs on hemoglobin levels
A unit of packed RBCs is highly concentrated with RBCs
For each unit of packed RBCs that is transfused into a patient (70 kg ro 154 lb) who is NOT actively bleeding, the patient's hemoglobin level can be expected to increase by 1 g/dL
1 mL of RBCs = 1 mg of iron
effects of transfusing packed RBCs on hematocrit levels
estimated that one unit of packed RBCs has a hematocrit level of approx 55-65%
For every unit of packed RBCs, the hematocrit can be expected to increase 3% in a patient (70-kg or 154 lbs) who is NOT actively bleeding
(transfusion therapy) special considerations for RBC components
Directed Donation
Blood from a specific donor drawn and set aside for a specific recipient
blood donation from a first-degree family member? → product must be irradiated for prevention of TA-GVHD
Family members more likely to share the same HLA haplotype
donated units are screened for infectious diseases and processed into packed RBC units
Community Directed Donations
Donor programs aim to treat patients diagnosed with hemoglobinopathies
e.g. sickle cell anemia (C, E, K matched), thalassemia
Benefits:
Decreased risk of alloimmunization, increased donor retention
(transfusion therapy) granulocytes
WBCs collected by apheresis and used to treat neutropenic patients with bacterial and/or fungal sepsis
These patients are typically resistant to antimicrobial therapy
Used as an interim therapy for patients expected to recover neutrophil production (1 × 1010 granulocytes)
Granulocyte units should always be irradiated to prevent TA-GVHD
Do NOT use microaggregate or leukocyte reduction filters
Both donor and recipient should be Rh and human leukocyte antigen (HLA) compatible
Crossmatch required when more than 2 mL of RBCs are present in the unit
(transfusion treatment of coagulopathies) FFP & transfusable plasma components
Fresh frozen plasma (FFP)
Indicated for patients with multiple coagulation deficiencies that occur in liver failure, DIC, vit K deficiency, warfarin overdose, massive transfusion and multiple-factor deficiencies
Transfusable Plasma Components
Cryoprecipitate-reduced plasma indicated for use for treating patients with thrombotic thrombocytopenic purpura (TTP), provides ADAMTS 13, and other coagulation factors
(transfusion treatment of coagulopathies) platelet products & cryoprecipitate
Platelet Products
Indicated for patients with thrombocytopenia
Prophylactic treatment for patients with platelet counts under 5,000 to 10,000/μL
Cryoprecipitate
Primarily used for fibrinogen replacement
(transfusion therapy) determining FFP effects
If separated from a whole blood unit, then the plasma is frozen or centrifuged for the purpose of removing platelets into a separate unit
Units are frozen w/in 8 hrs of collection to preserve all coag factors (FFP)
Units frozen after 8 hours BUT within 24 hours = PF24
Fresh-frozen units thawed in the following:
37°C water bath / FDA-approved microwave
Considerations for plasma
1 unit of plasma increases clotting factors by 10%
2 units usually sufficient to increase clotting factor activity by 15% to 20% in a pt with diminished clotting capacity
(transfusion therapy) platelet components
May be manufactured from WB or apheresis collection
Platelet transfusion indicated in the following conditions:
Decreased platelet production
Increased platelet destruction
Platelet dysfunction
Medications (e.g., aspirin) can alter or interfere with platelet function
Platelet Refractoriness – recipients no longer exhibit the expected increase in platelet numbers after infusion of a platelet product
Commonly caused by alloimmunization directed towards HLA or platelet antigens
effects of transfusing WB-derived platelet concentrate
will increase the platelet count by 5,000-10,000/uL in an adult patient
Considered a prophylactic dosage to prevent spontaneous bleeding (contains a minimum of 5.5 x 1010 platelets)
A platelet count of 50,000/μL deemed sufficient for most surgeries
Transfusion of whole blood-derived platelets will increase the platelet count by 50,000/μL in pediatric patients
how much platelet concentrate is needed to prevent spontaneous bleeding?
Pool of 4-6 platelet concentrates (random donor platelets) OR a single donor apheresis unit (contains min 3.0 x 1011 platelets) is sufficient to prevent spontaneous bleeding in a normal sized adult
1 single donor apheresis unit = 4-6 random donor platelet concentrates; therefore, it should increase the platelet count by 20,000 – 60,000/μL in an adult patient (approximately 70 kg)
transfusion considerations for platelet products
In the absence of platelet destruction or consumption, platelet transfusions may occur every 3 to 5 days
Daily administration of platelets may be necessary if platelet destruction or consumption is observed in a patient
In the presence of platelet destruction or consumption, the platelet count may not improve
(transfusion therapy) cryo, fibrin glue, platelet gel
Cryoprecipitate
Manufactured from FFP—has fibrinogen, factor VIII, factor XIII, VWF, and fibronectin
Used to treat hemophilia A and source of fibrinogen (<100 mg/dL)
Fibrin Glue
Sealant in surgical procedures for tight closure of wound
Platelet Gel
Intraoperative combination of autologous platelets, WBC-rich plasma, calcium chloride, and thrombin
Stimulates coagulation and healing
(plasma fractionation products) albumin, RHIG, IVIG
Albumin
Used as a volume replacement in trauma, shock, burns, and therapeutic plasma exchange
Rh immune globulin (RhIG)
Administered to prevent alloimmunization against D antigen
Intravenous immune globulin (IVIG)
A product composed of IgG antibodies direct against many antigens
Commonly used to treat hypogammaglobulinemia
(plasma fractionation products) hyperimmune globulins & coag factor concentrates
Hyperimmune globulins
Specific immune globulins prepared from donor plasma with high titers--used as passive immunity for MMR, CMV, tetanus, varicella, Hep B and others
Coagulation factor concentrates
Used to prevent or treat bleeding episodes in patients with coagulation deficiencies
Factor VIII, Factor IX, Factor XIII, Factor VII, fibrinogen and more
(transfusion therapy; pharmaceuticals) DDVAP & vitamin K
Desmopressin (DDAVP)
reduces or prevents bleeding in patients with mild hemophilia A or type I von Willebrand disease
Vitamin K
can reverse the effects of oral anticoagulants overdose
(transfusion therapy; pharmaceuticals) erythropoietin & thrombopoietin
Erythropoietin
recombinant EPO used to treat anemia in patients with renal failure
Thrombopoietin
recombinant TPO stimulates platelet production in patients suffering from bone marrow suppression that has caused thrombocytopenia
(transfusion therapy; pharmaceuticals) colony stimulating factors & plerixafor
Colony-stimulating factors
HPC donors and granulocyte donors receive this medication prior to donation
Used for treatment of neutropenia after chemotherapy in cancer patients
Plerixafor
used as a stem cell mobilizer for patients with non-Hodgkin’s lymphoma or multiple myeloma
(transfusion therapy; pharmaceuticals) antifibrinolytics & recombinant factor VIIa
Antifibrinolytics
inhibits fibrinolysis and commonly used during cardiac surgery to reduce bleeding
Recombinant factor VIIa
used as a supplement for patients with Factor VII deficiency and treating patients with Hemophilia A/B
massive transfusion (general)
Replacement of one or more total blood volumes within 24 hours or about 10 units of blood in adult
critical first step: establishing intravenous access
Crystalloid solutions may be used to restore circulating systemic volume to avoid circulatory collapse
Infusion of FFP, platelets, cryoprecipitate aid in restoring hemostatic function
Rapid infusers and blood warmers commonly used
Clinical status of patient and inventory of transfusion service assessed regularly to evaluate any changes
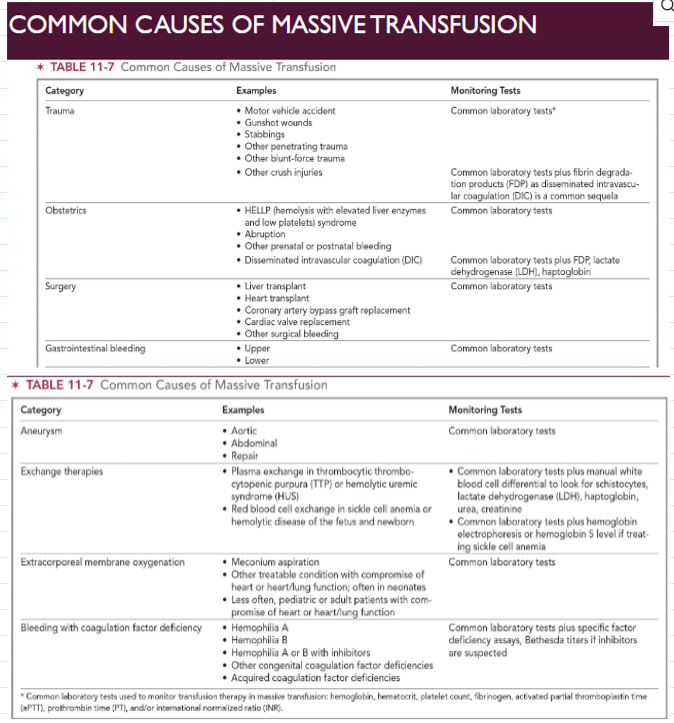
transfusion committee
Consists of members representing both laboratory and clinical areas
Review of all elements required by Standards
Ordering practices, patient identification, adverse events, near-misses, inventory management, blood administration policies
Cases of massive transfusion often reviewed by transfusion committee
Proper patient identification, appropriate blood usage, patient needs met
Routinely involved in drafting or reviewing emergency preparedness plans
(transfusion therapy) testing for anti-CMV
In immune-suppressed individuals, CMV infection can cause debilitating effects and even death
The two methods to decrease risk of transfusion-associated CMV transmission
Leukoreduction
Testing blood donors for antibodies to CMV to provide CMV-negative units
(transfusion therapy) hemoglobin S testing
RBCs selected for transfusion to a patient diagnosed with SCD (or neonates) should test negative for hemoglobin S
Screening methods include use of the Hgb solubility test
e.g., Sickledex
RBC products from sickle cell trait donors should not be transfused into a patient with SCD
Phenotypically matched for C, E, K antigens
Considerations for use of autologous blood
(modifications of cellular components) irradiation
Used to prevent A-GVHD in recipients (immunocompromised)
Viable transfused T lymphocytes replicate in recipient, recognize recipient as foreign, then a destructive immune response is mounted against recipient's body
Gamma irradiation disrupts DNA in WBC nuclei
Destroys WBCs' ability to replicate
Standard dose: 2,500 cGy (centigray)
RBCs, PLT, and granulocyte products contain WBCs
(modifications of cellular components) freezing
Blood from donors with rare RBC phenotypes stored frozen for up to 10 years
Used for autologous or allogeneic transfusion
Each unit is phenotyped prior to storage and frozen with a cryoprotective agent (e.g., 20% or 40% glycerol)
Deglycerolization is performed to wash the cryoprotective agent off from frozen RBCs prior to transfusion
open system = 24 hours expiration
closed system = good for 2 weeks
Frozen platelets can be stored for up to 2 years
(modifications of cellular components) washing
Washing RBC units eliminates 85% of WBCs, 15% of RBC mass, and 99% of plasma
Process performed as an open system = seal of unit is breached
Washed RBCs expire in 24 hrs
Washed PLTs expire in 4 hrs
Closed system: washed RBCs expire in 2 weeks
Patients with severe allergic reactions to blood products, e.g., transfused to IgA deficient patients who have antibodies to IgA
Rare donor units, autologous units