W2L1: Non-Covalent Bonding and Resonance
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Structure of an atom
Protons are positively charged
Neutrons have no charge
Electrons are negatively charged
Atomic number = # of protons
Atomic number of carbon = 6
Neutral carbon has six protons and six electrons
Isotope
Isotopes have the same atomic number but different mass numbers
What makes carbon so special
Atoms to the left of carbon give up electrons
Atoms to the right of carbon accept electrons
Carbon shares electrons
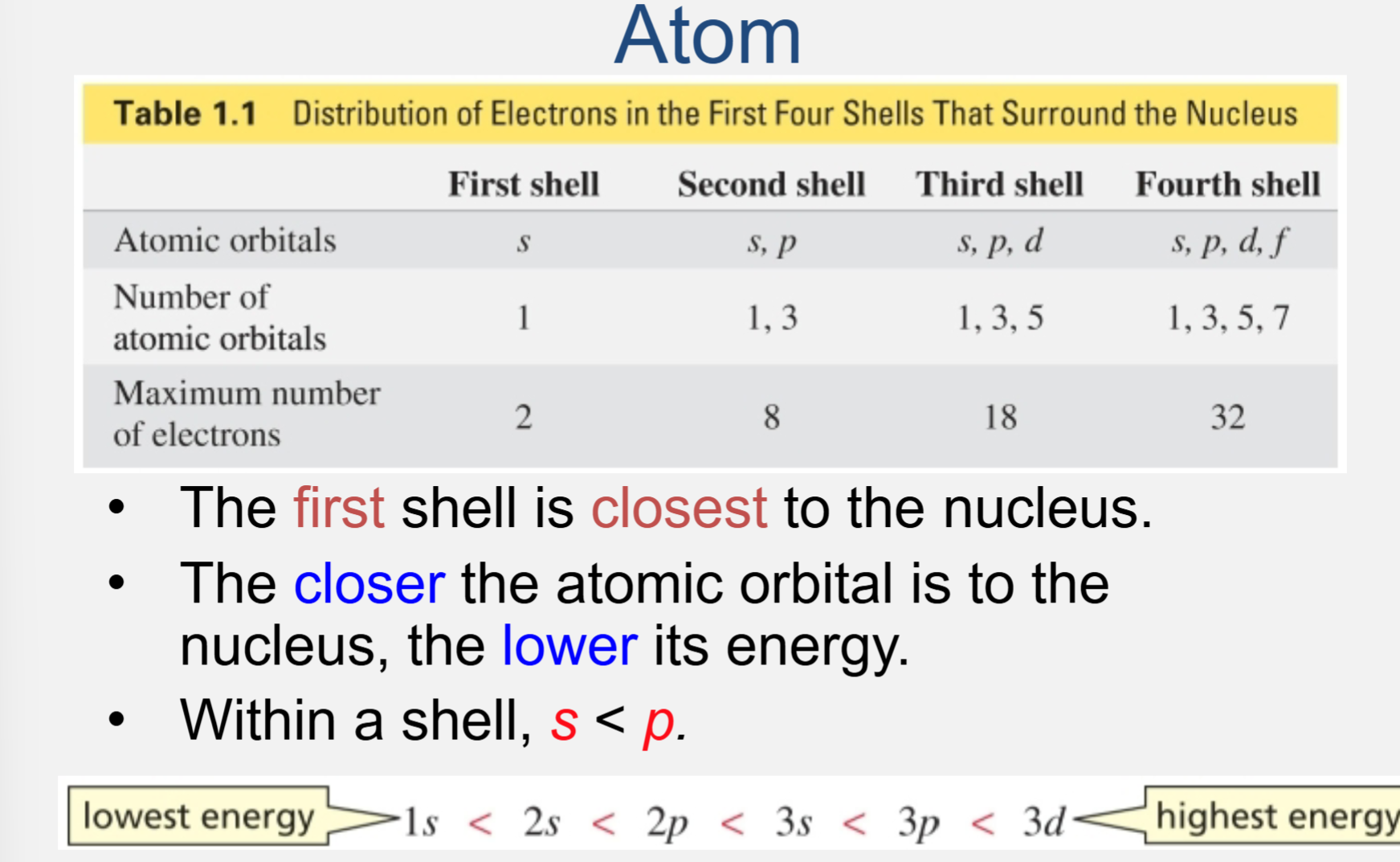
Distribution of electrons in an atom
The first shell is closest to the nucleus
The closer the atomic orbital is to the nucleus, the lower its energy
Within a shell, s < p
Aufbau principle
An electron goes into the atomic orbital with the lowest energy
Valence shell, lone pair
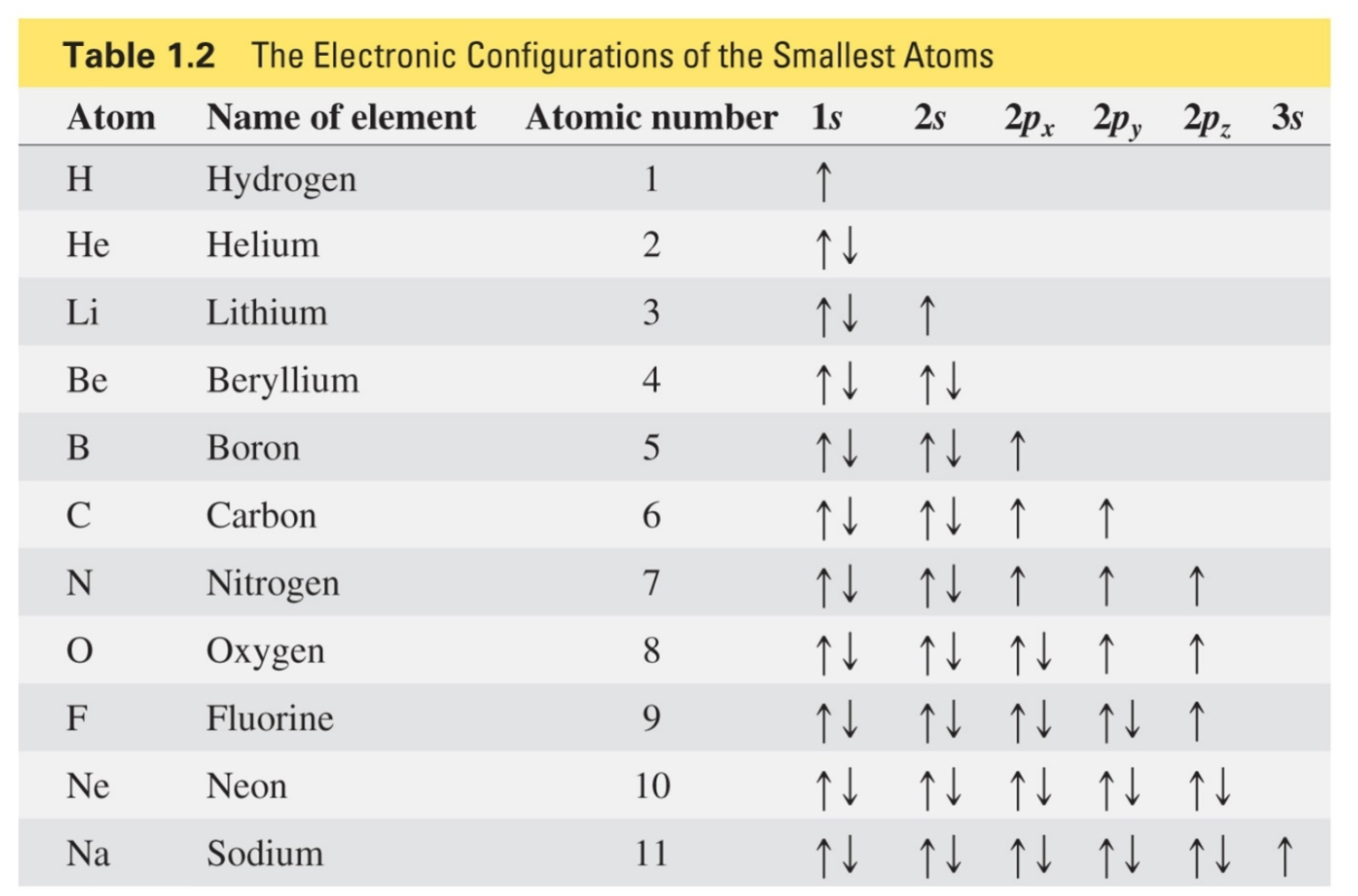
Pauli exclusion principle
No more than 2 electrons can be in an atomic orbital
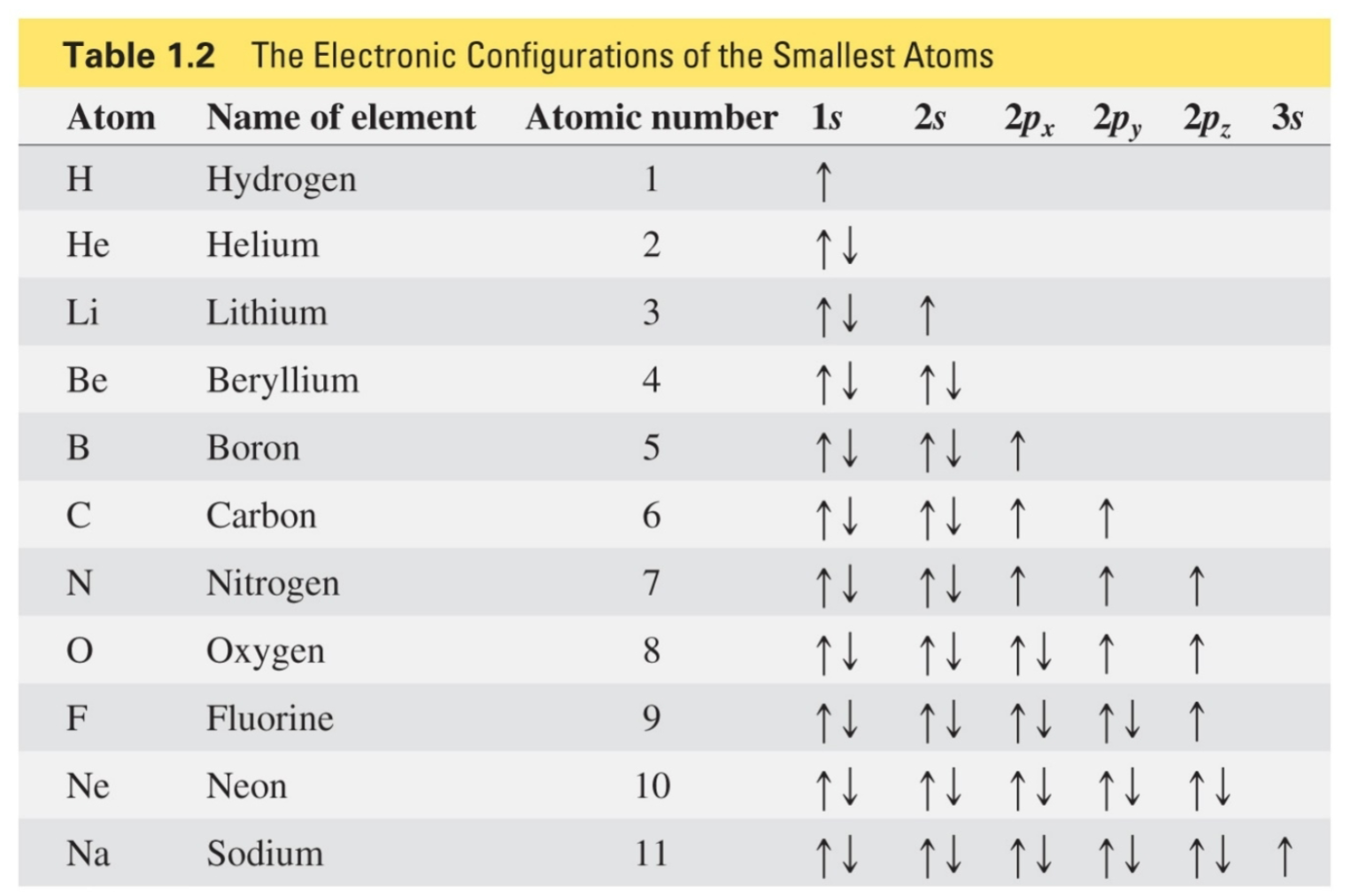
Hund’s rule
An electron goes into an empty degenerate orbital rather than pairing up
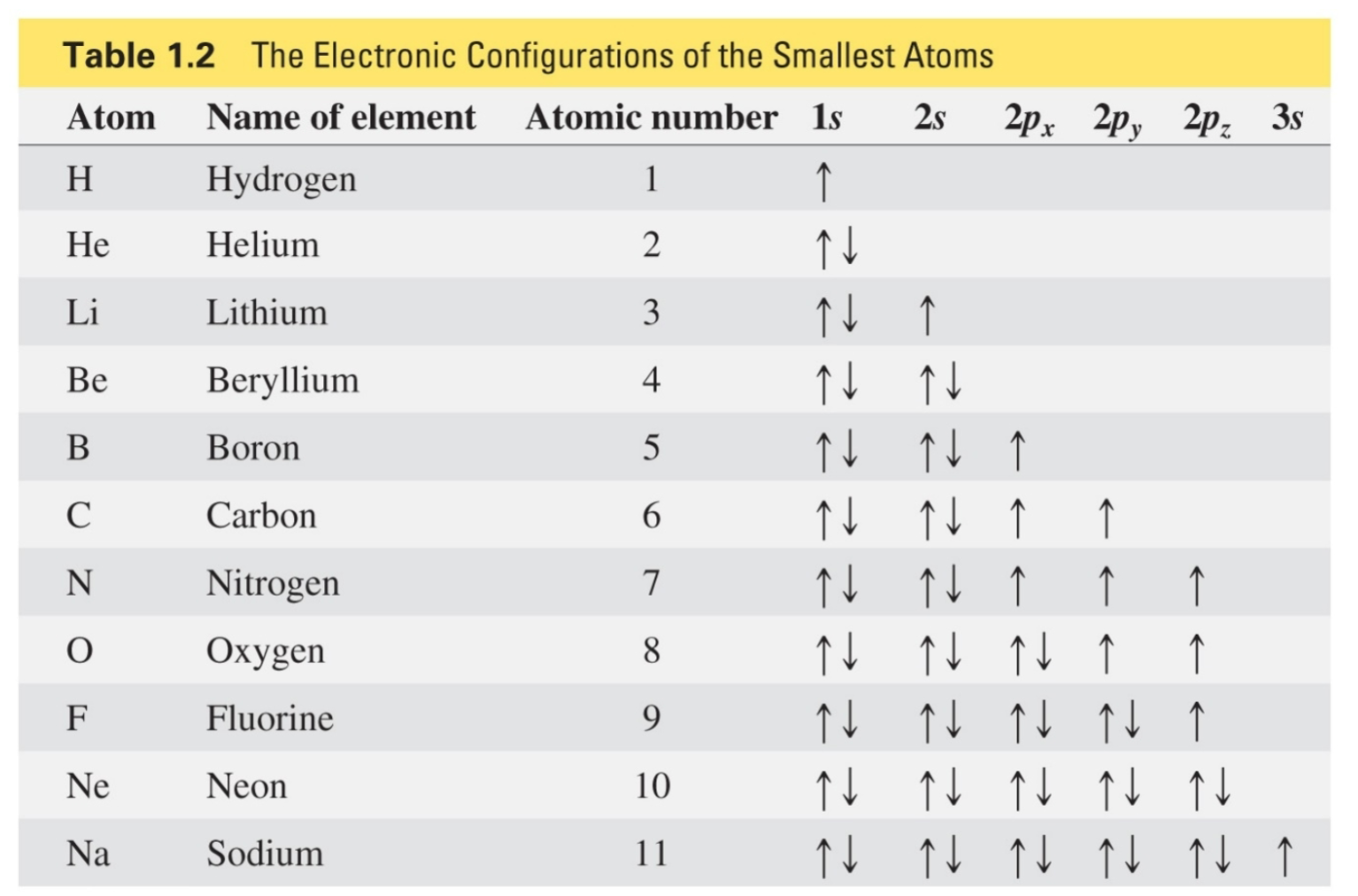
Atoms on the left side of periodic table
Lose an electron
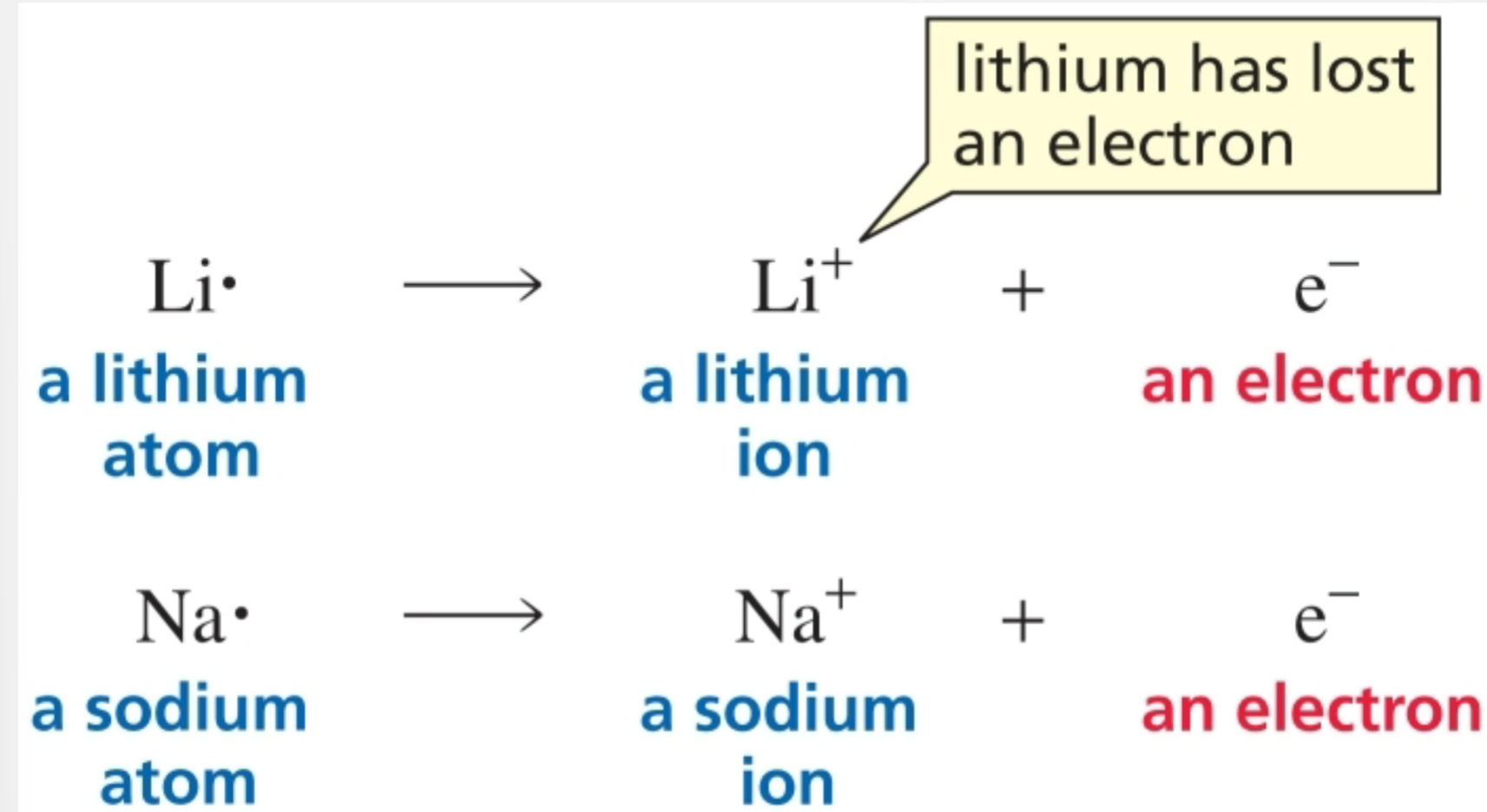
Atoms on the right side of the periodic table
Gain an electron
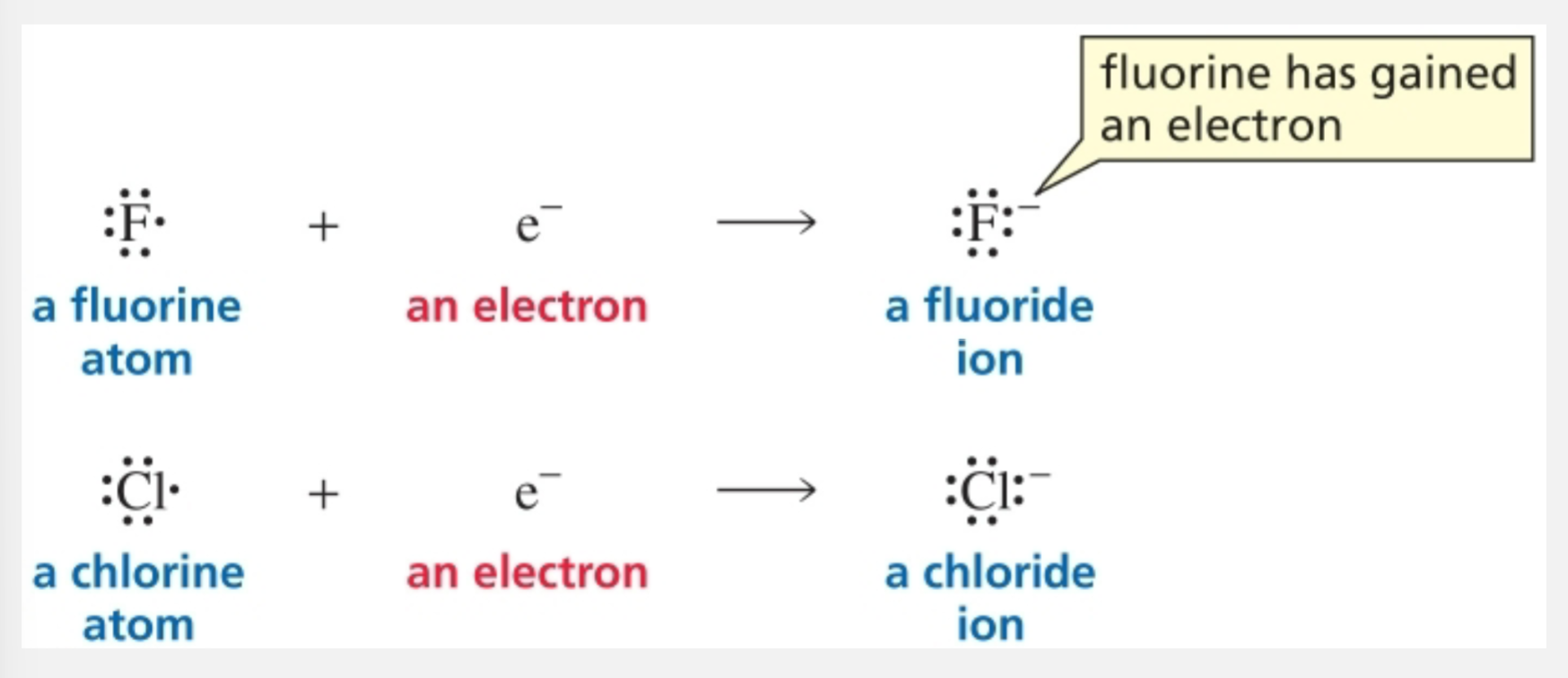
Hydrogen and electrons
Hydrogen can lose or gain an electron
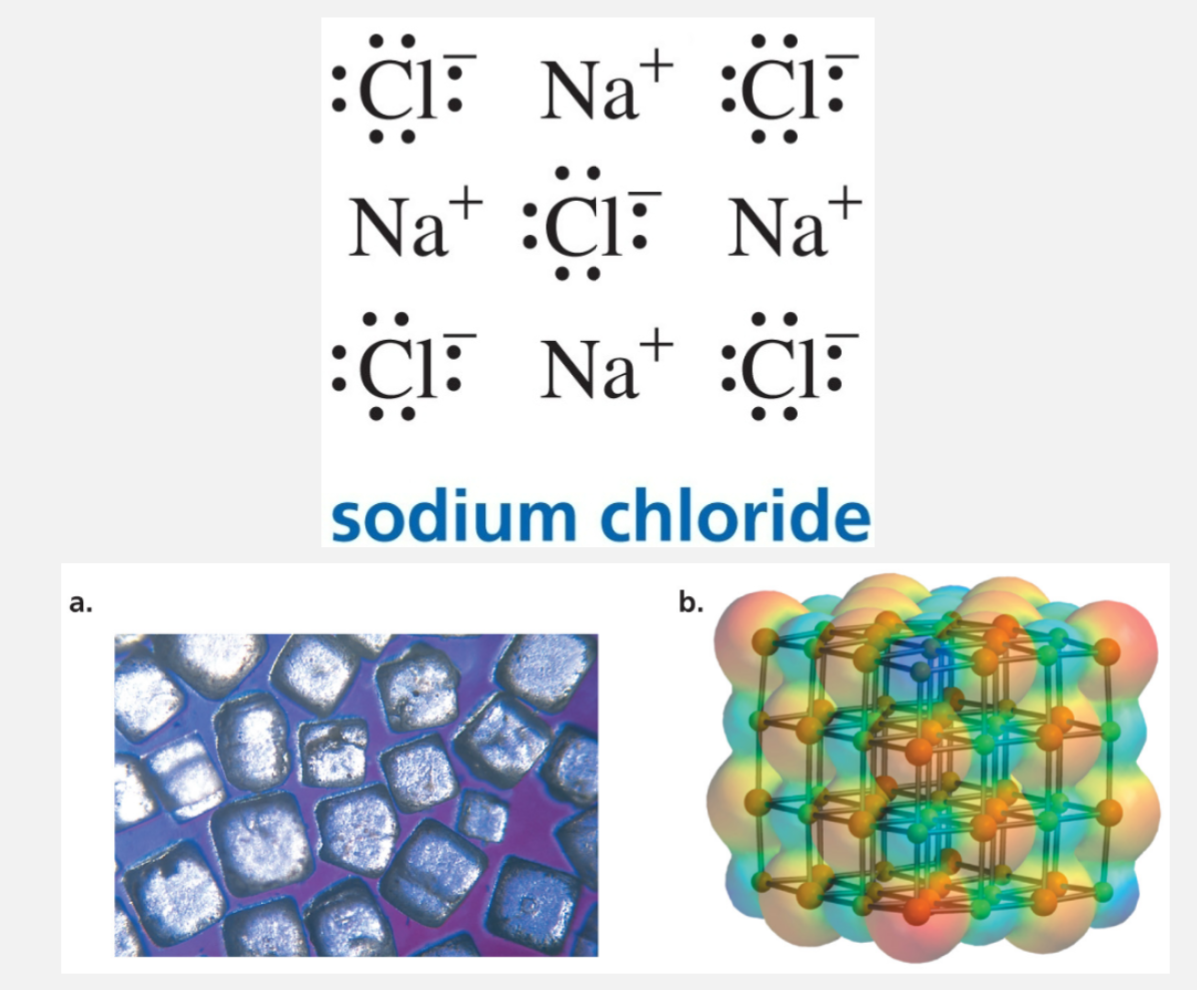
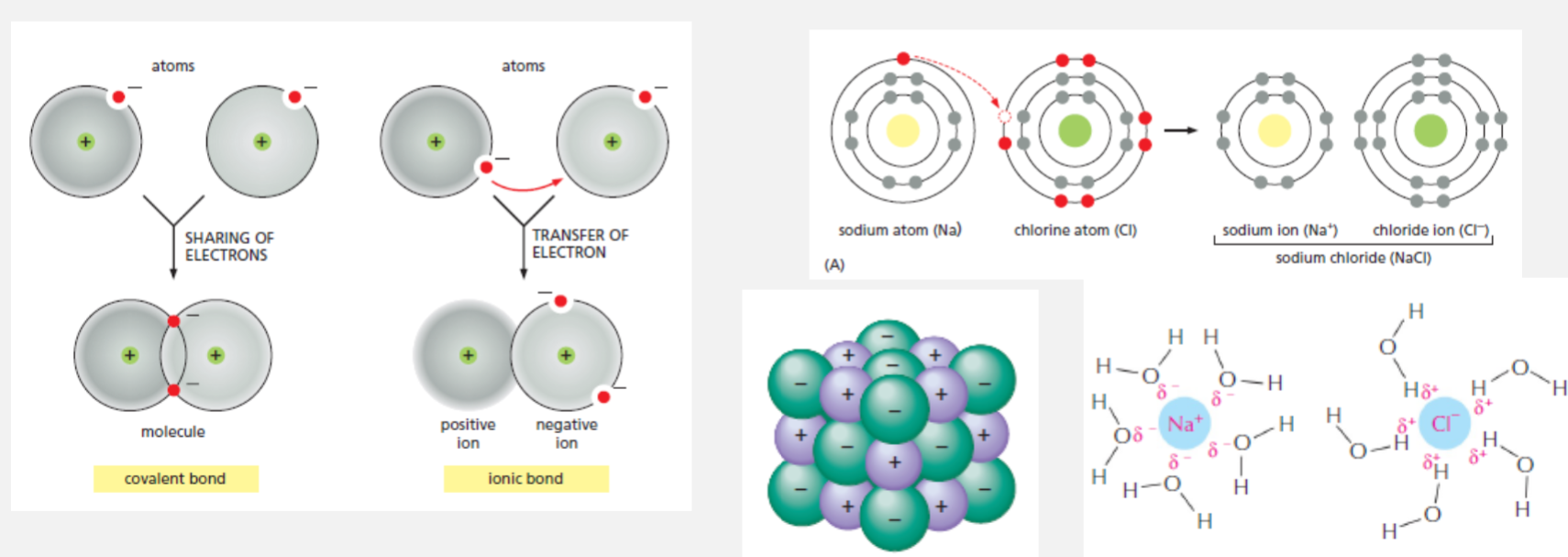
Forming ionic bonds
An ionic bond is formed by the attraction between ions of opposite charge
Ionic bonds
Ionic bonds normally form between atoms that have a big difference in their electronegativities
No directionality - the attraction is purely electrostatic: any positive ion is attracted equally to any nearby negative ion, regardless of angle or orientation
Forming covalent bonds
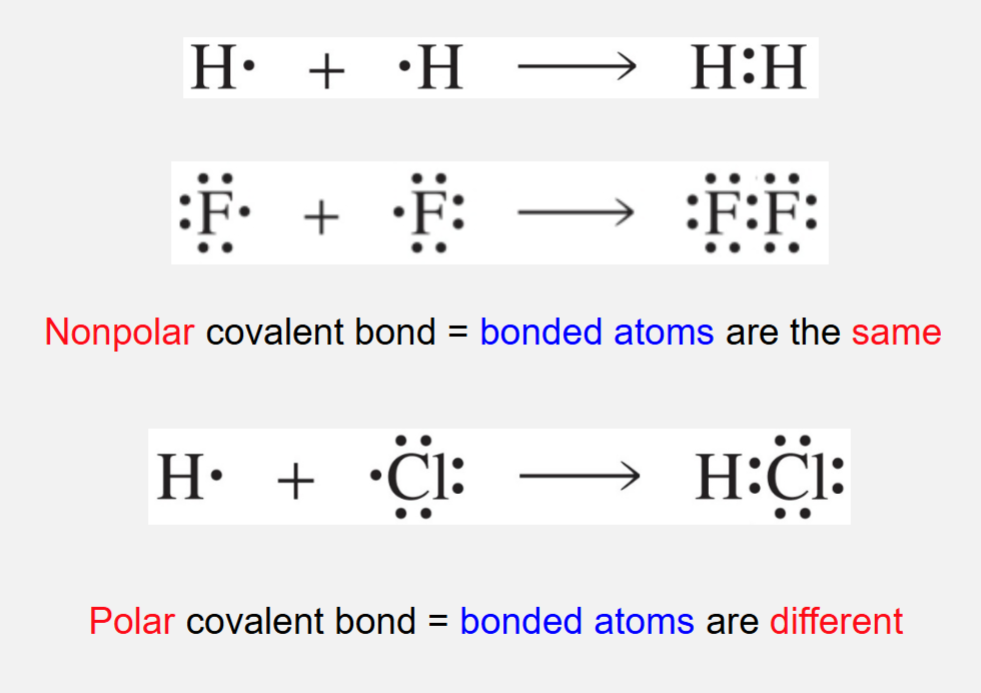
Formed by sharing electrons
Non polar covalent bond = bonded atoms are the same
Polar covalent bond = bonded atoms are different
Electronegativity
The tendency of an atom in a molecule to attract electrons to it
Polar covalent bonds
A polar covalent bond is a covalent bond in which the electron pair is not shared equally between the 2 atoms
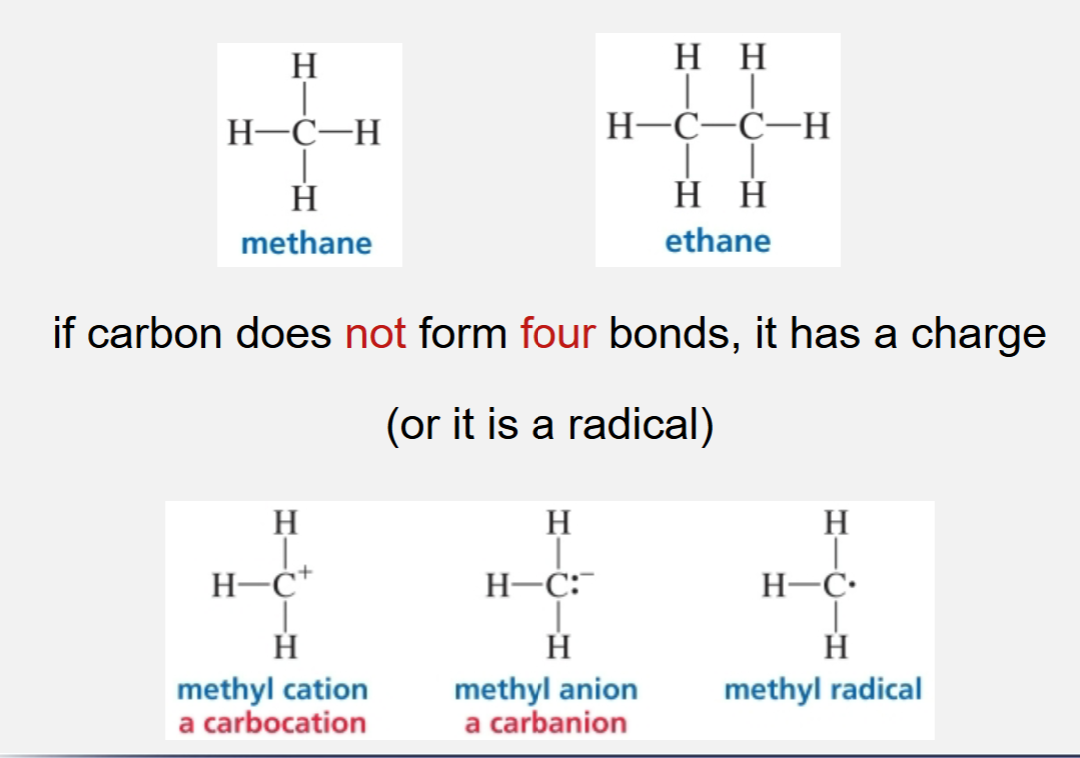
Neutral carbon forming bonds
Neutral carbon can form 4 bonds and it has a charge (or it is a radical)
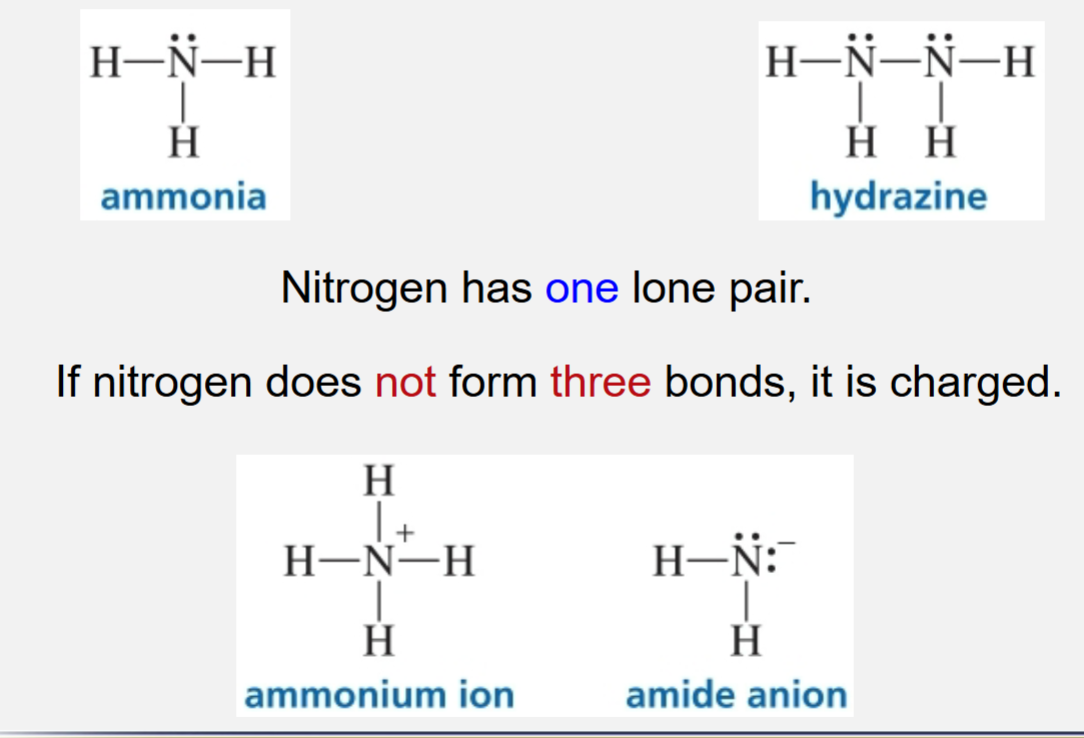
Neutral nitrogen forming bonds
Neutral nitrogen forms 3 bonds
If nitrogen does not form 3 bonds, it is charged
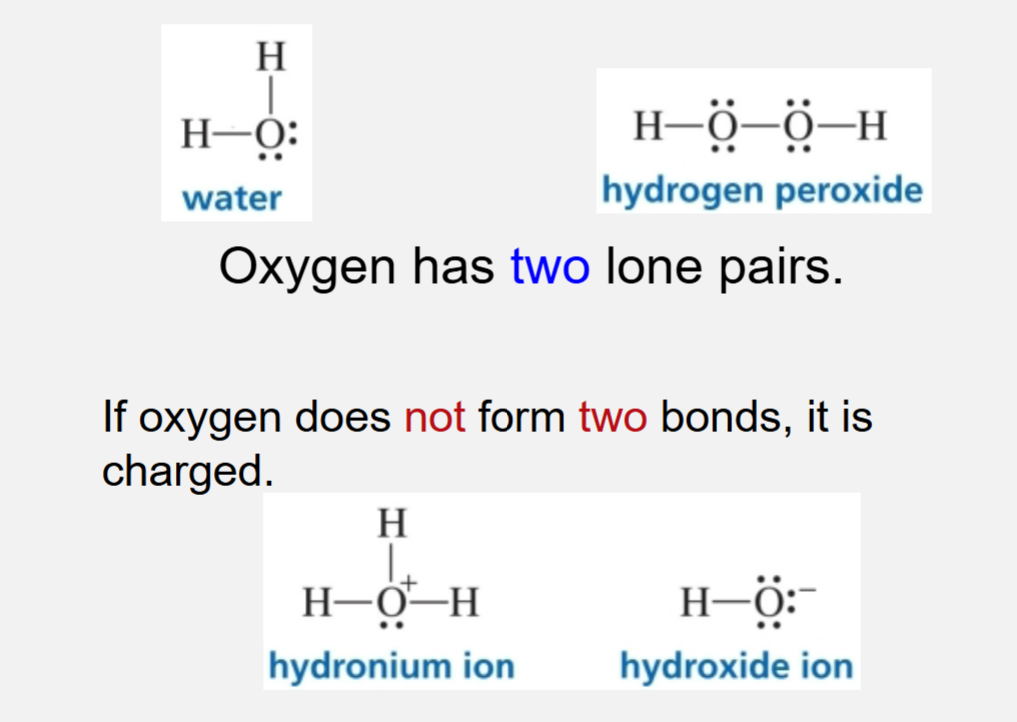
Neutral oxygen
Neutral oxygen forms 2 bonds
Oxygen has 2 lone pairs
If oxygen does not form 2 bonds it is charged
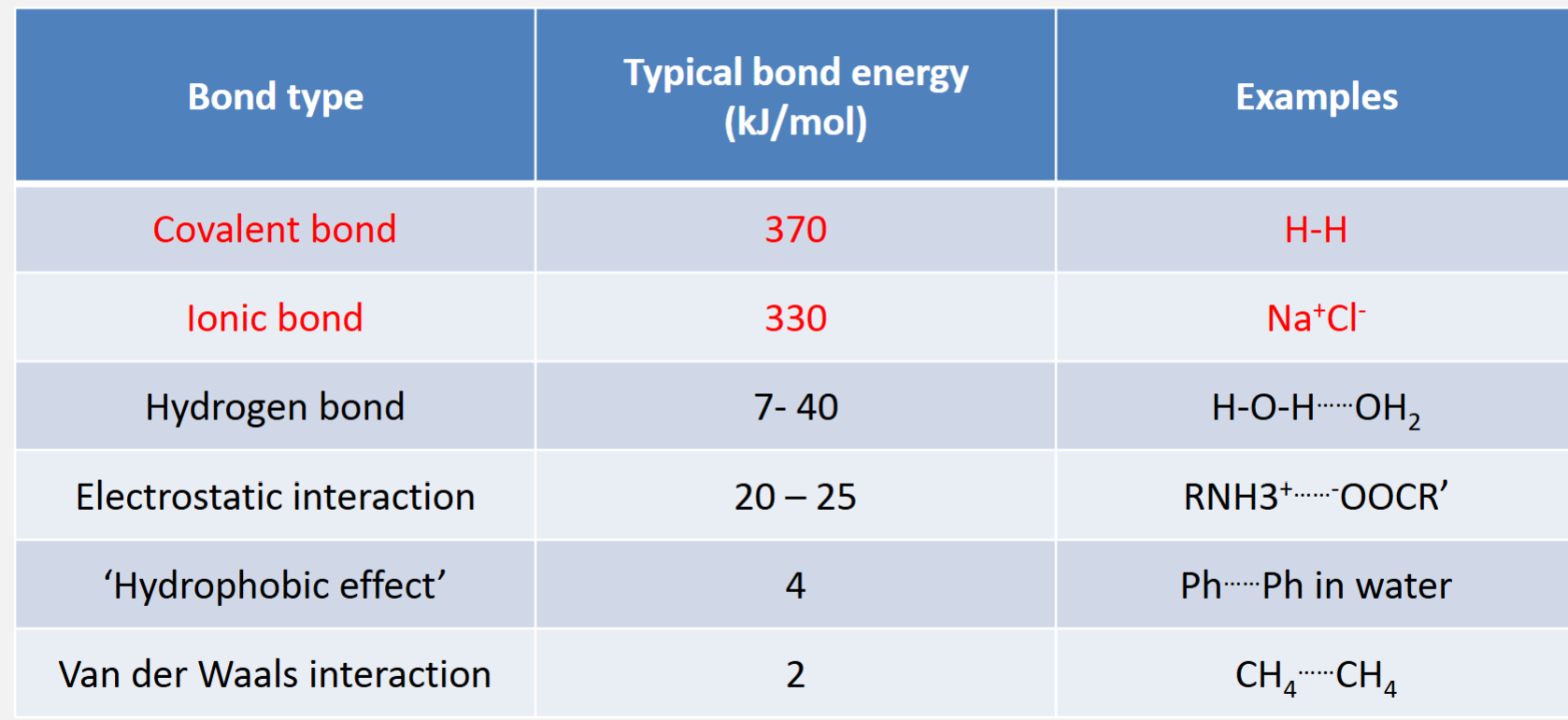
Non-covalent interactions
Arises due to electrostatic interactions between molecules or atoms that are not bonded together (not sharing electrons via a covalent bond)
Much weaker than a covalent bond but occurs very frequently
Includes: Van der Waals interactions, hydrogen bonding, ionic interactions, hydrophobic effect
Van der Waals interactions
A weak interaction due to fluctuating electrical charges between molecules
Strength is strongly distance dependent - Van der Waals radius
Hydrogen bond
A special type of dipole-dipole interaction between H and a non-bonding heteroatom (e.g. O or N)
Stronger than van der Waals interactions, but weaker than covalent bonds
Strongly dependent on geometry of atoms involves
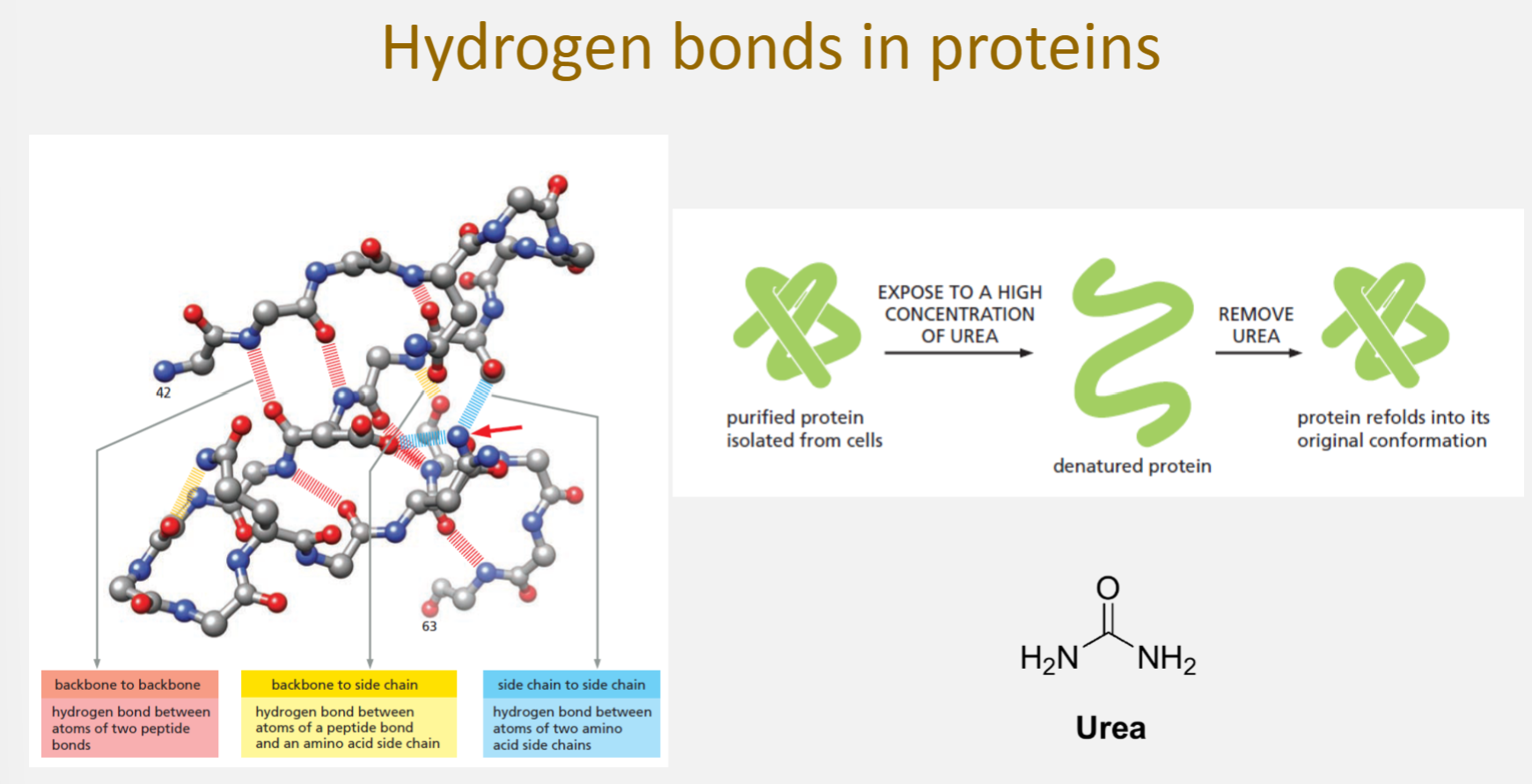
Hydrogen bonds in proteins
Backbone to backbone (pink): hydrogen bonds between atoms of two peptide bonds
Backbone to side chain (yellow): hydrogen bonds between a peptide bond atom and an amino acid side chainSide chain to side chain (blue): hydrogen bonds between atoms of two amino acid side chains
These bonds stabilize secondary and tertiary protein structures
Urea denaturation
Purified protein isolated from cells → normally folded with correct hydrogen bonds
Expose to high concentration of urea → protein becomes denatured (unfolded) because urea disrupts hydrogen bonds
Remove urea → protein can refold into its original conformation (renaturation) because the sequence of amino acids (primary structure) contains all the information needed for correct folding
Urea can form hydrogen bonds with polar groups in the protein, competing with the protein’s internal hydrogen bonds and leading to unfolding.
Urea denatures proteins by interfering with these hydrogen bonds, but removal of urea allows the protein to refold correctly
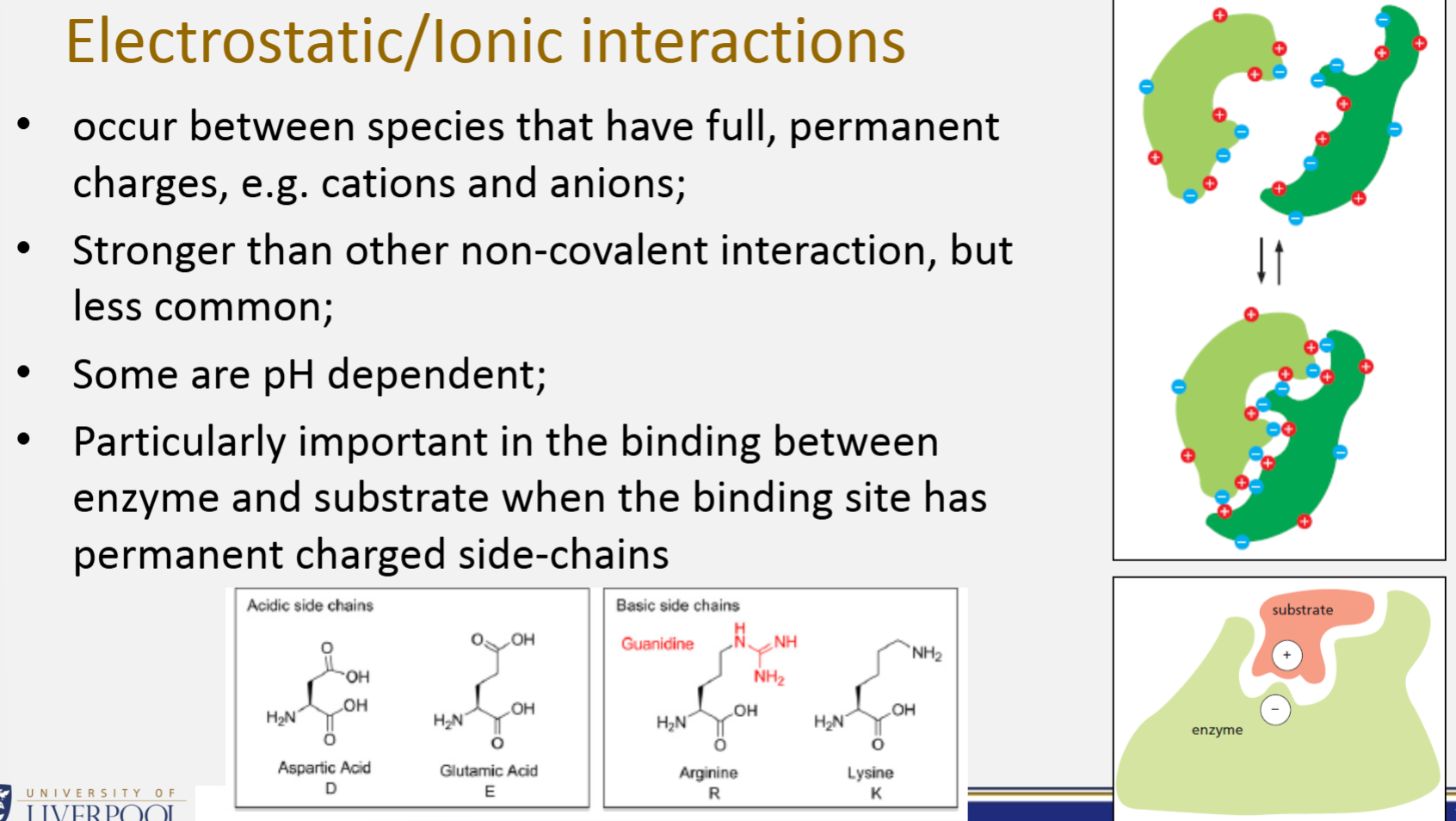
Electrostatic/Ionic interactions
Occur between species that have full, permanent charges, e.g. cations and anions
Stronger than other non-covalent interaction, but less common
Some are pH dependent
Particularly important in the binding between enzyme and substrate when the binding site has permanent charged side-chains
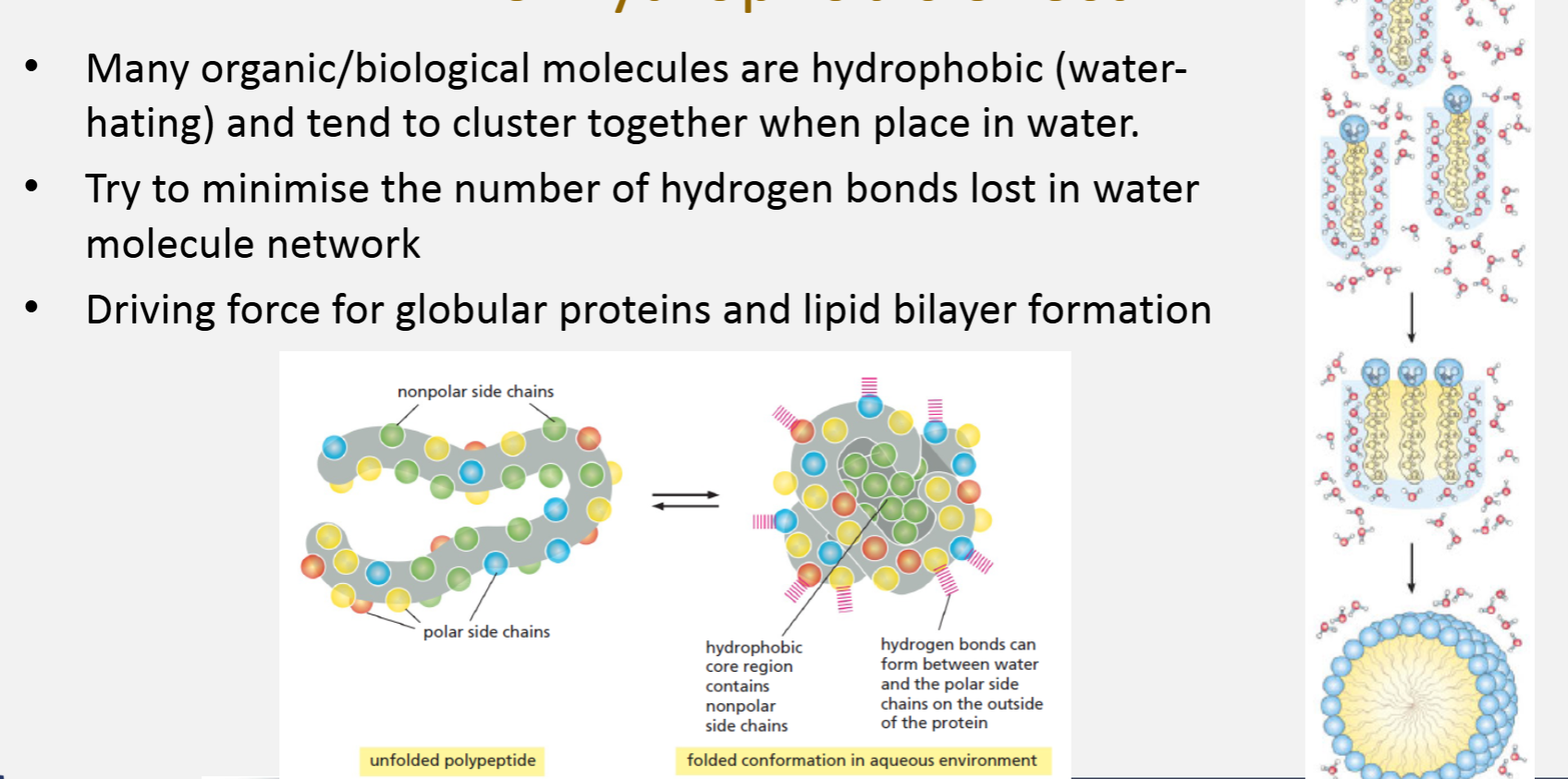
The ‘hydrophobic effect’
Many organic/biological molecules are hydrophobic (water-hating) and tend to cluster together when place in water
Try to minimise the number of hydrogen bonds lost in water molecule network
Driving force for globular proteins and lipid bilayer formation
Comparison between non-covalent interactions
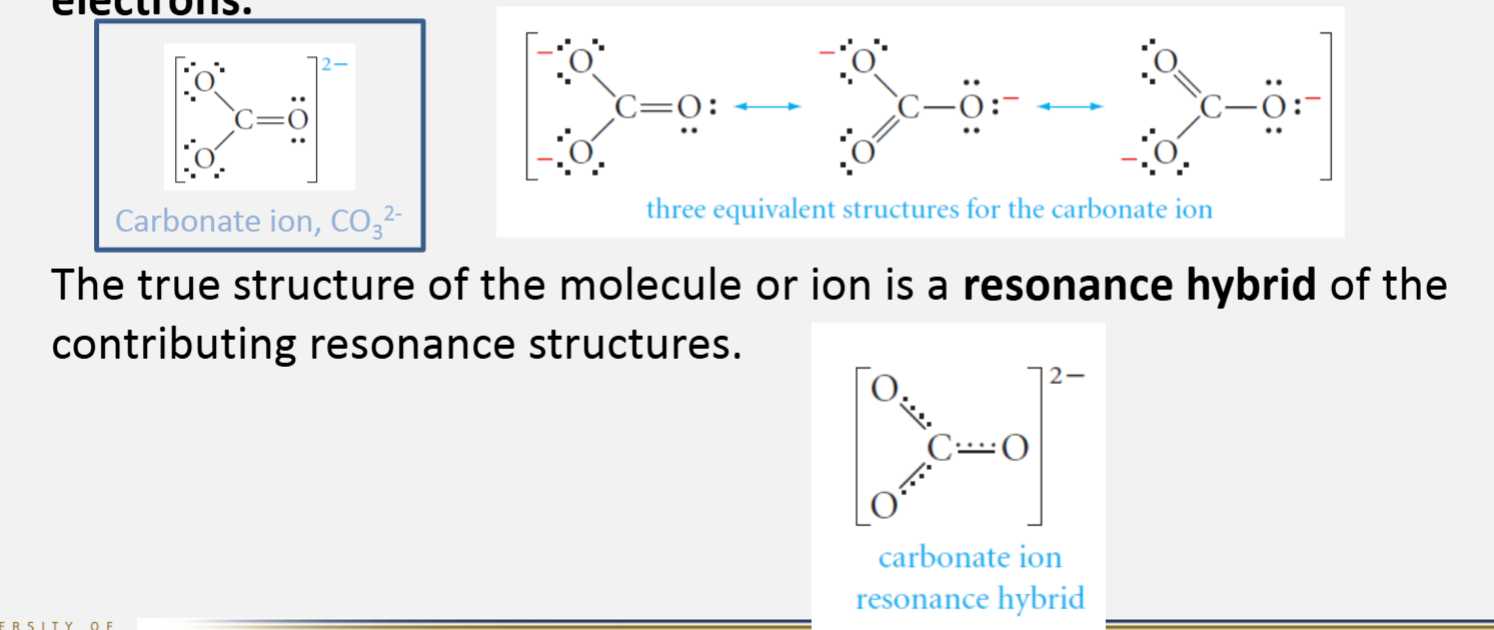
Resonance
Resonance structures of a molecule or ion are two or more structures with identical arrangements of the atoms but different arrangements of the electrons
The true structure of the molecule or ion is a resonance hybrid of the contributing resonance structures
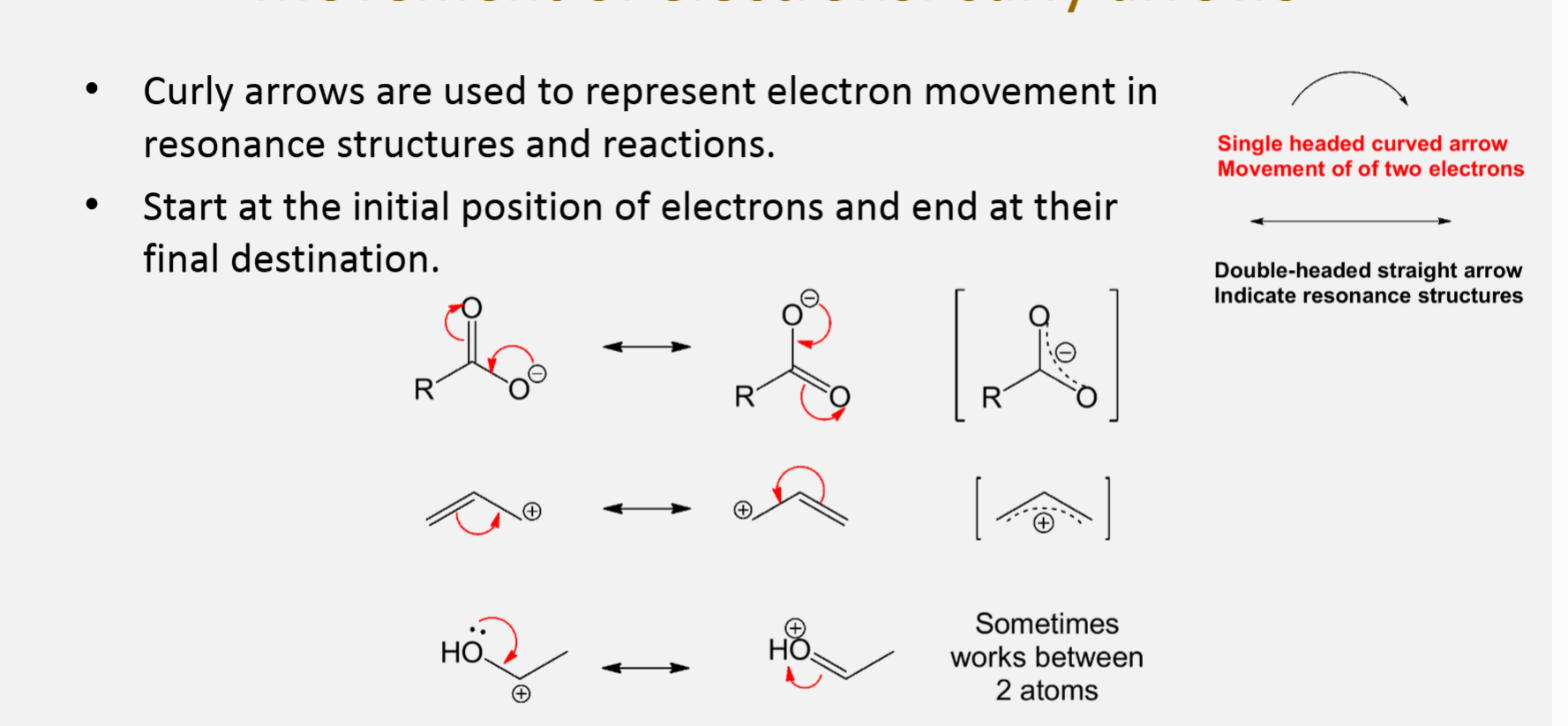
Movement of electrons
Curly arrows are used to represent electron movement in resonance structures and reactions
Start at the initial position of electrons and end at their final destination
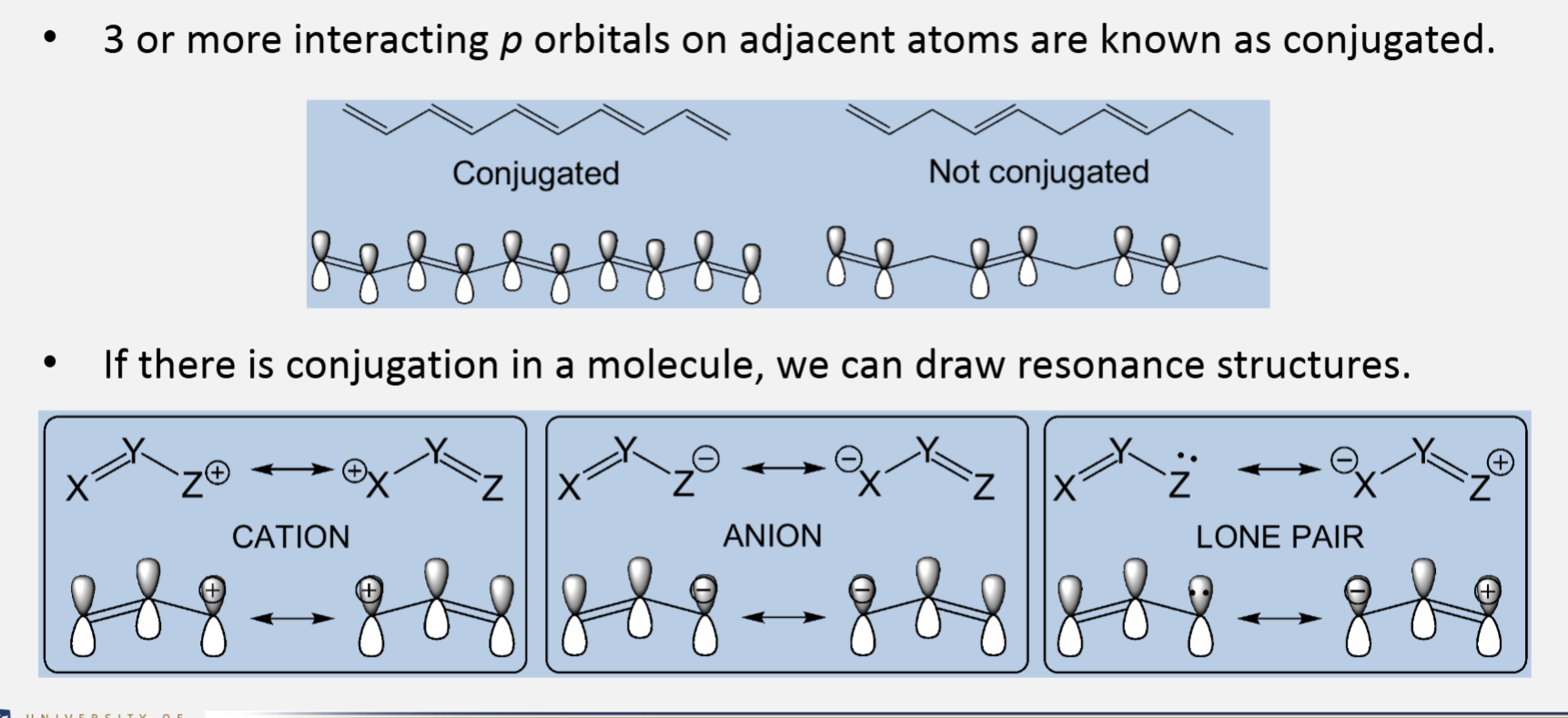
Conjugation
3 or more interacting p orbitals on adjacent atoms are known as conjugated
If there is conjugation in a molecule, we can draw resonance structures
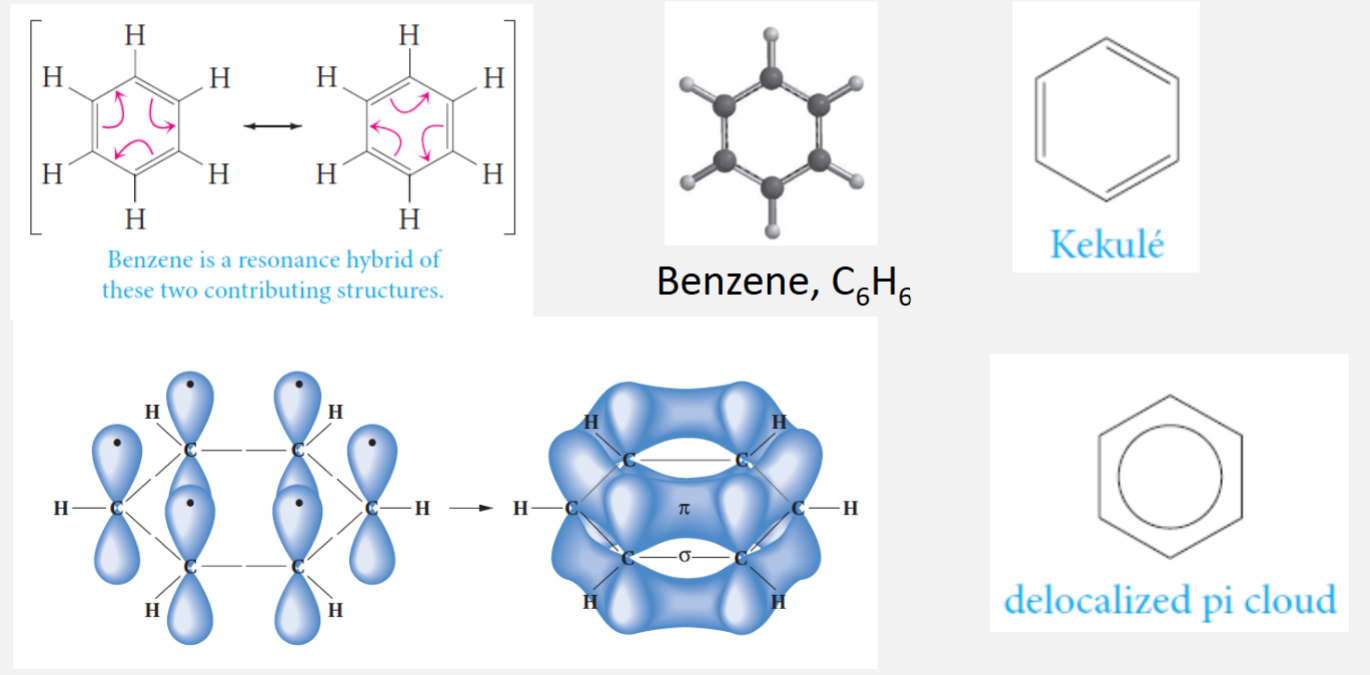
Benzene structure
Benzene is a very stable molecule (because of conjugation)
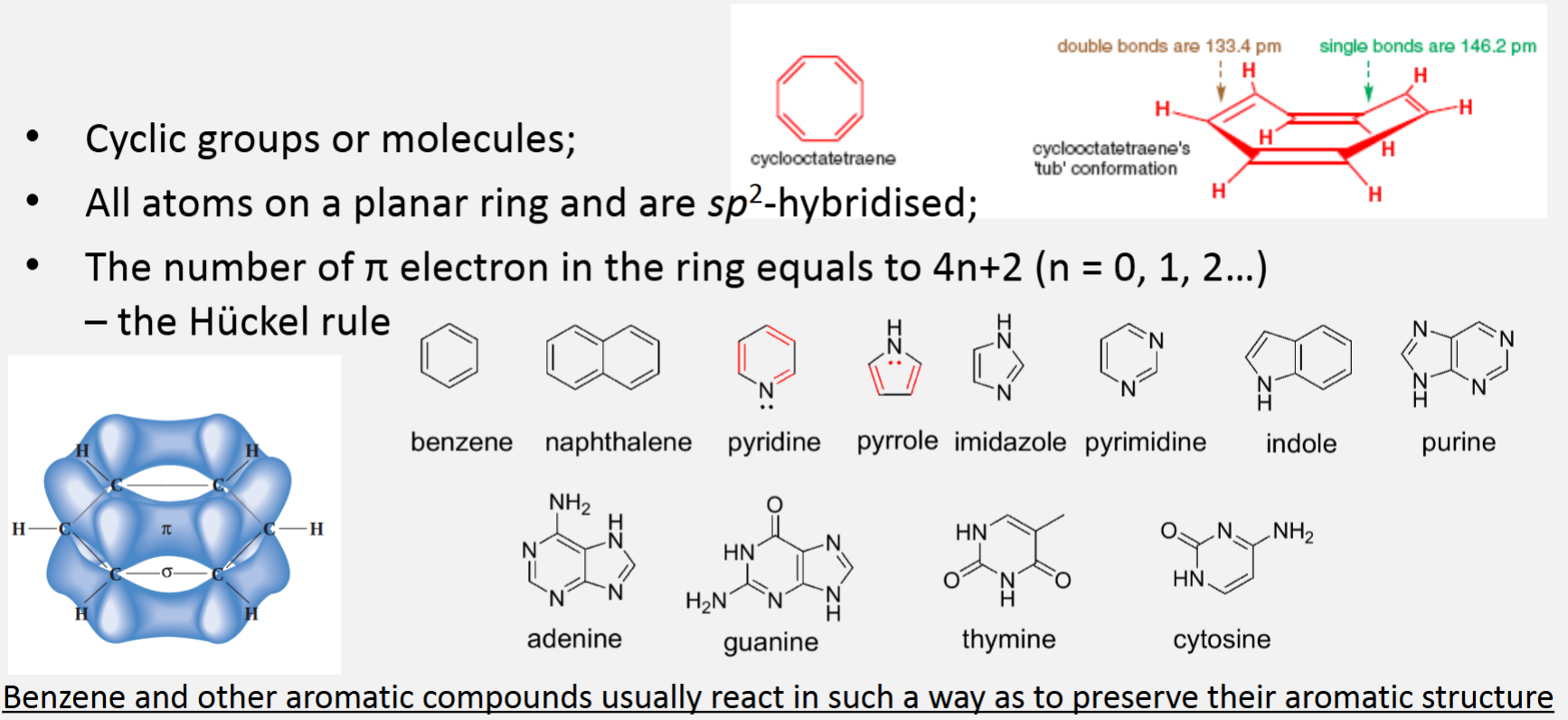
Aromaticity and aromatic compounds
Cyclic groups or molecules
All atoms on a planar ring and are sp2-hybridised
The number of π electron in the ring equals to 4n+2 (n = 0, 1, 2...) - the Hückel rule
Benzene and other aromatic compounds usually react in such a way as to preserve their aromatic structure
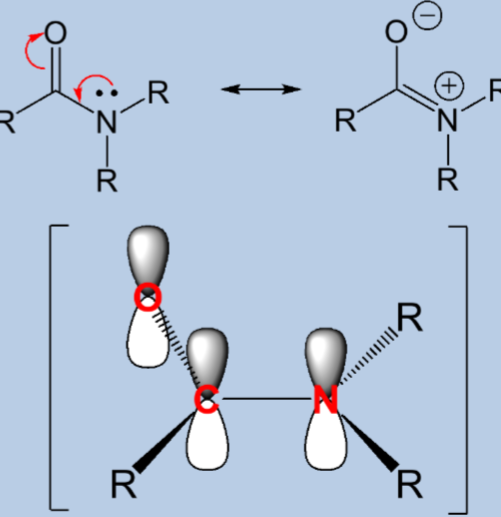
The resonance structures of amide
The amide is the functional group for peptides. The resonance ability of amide results in some key chemical and structural characters of peptides
Neutral structure clearly favoured
Delocalisation strengthens the C-N bond as it is partially a double bond
Since the lone pair is being used, it can’t act as a base or nucleophile
The O-C-N are all planar (sp2 hybridised)
Peptide bond is rigid