1- Introduction to organic chemistry
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
The actual number of atoms of each element in a molecule
What is meant by the molecular formula?
C2H6
Ethane molecular formula
The simplest whole number ratio of atoms of each element in a compound
What is meant by the empirical formula?
CH3
Ethane empirical formula
Ethane skeletal
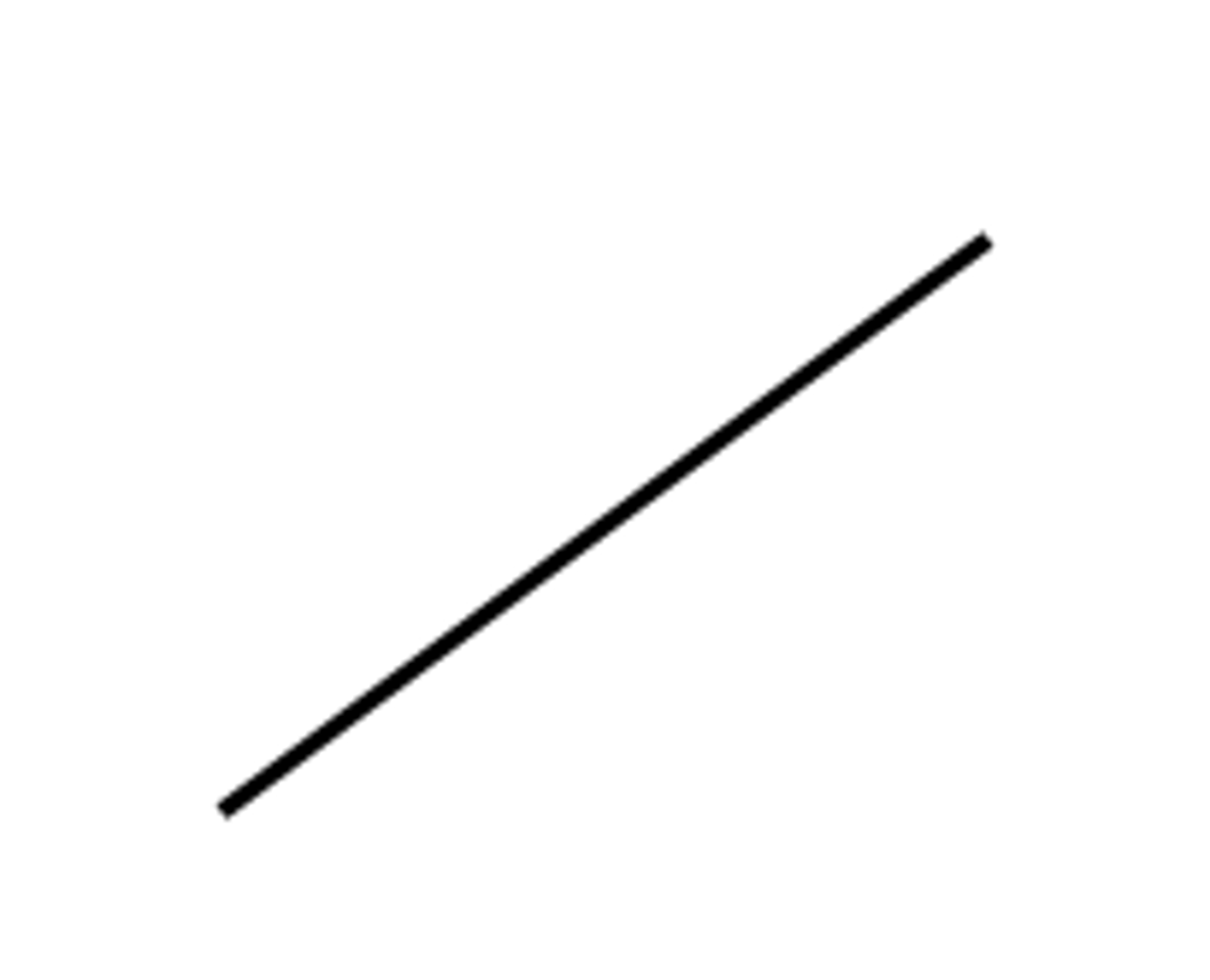
Each line is a carbon atom, starting from a bottom position
How does the skeletal formula represent molecules?
.
Ethane displayed formula
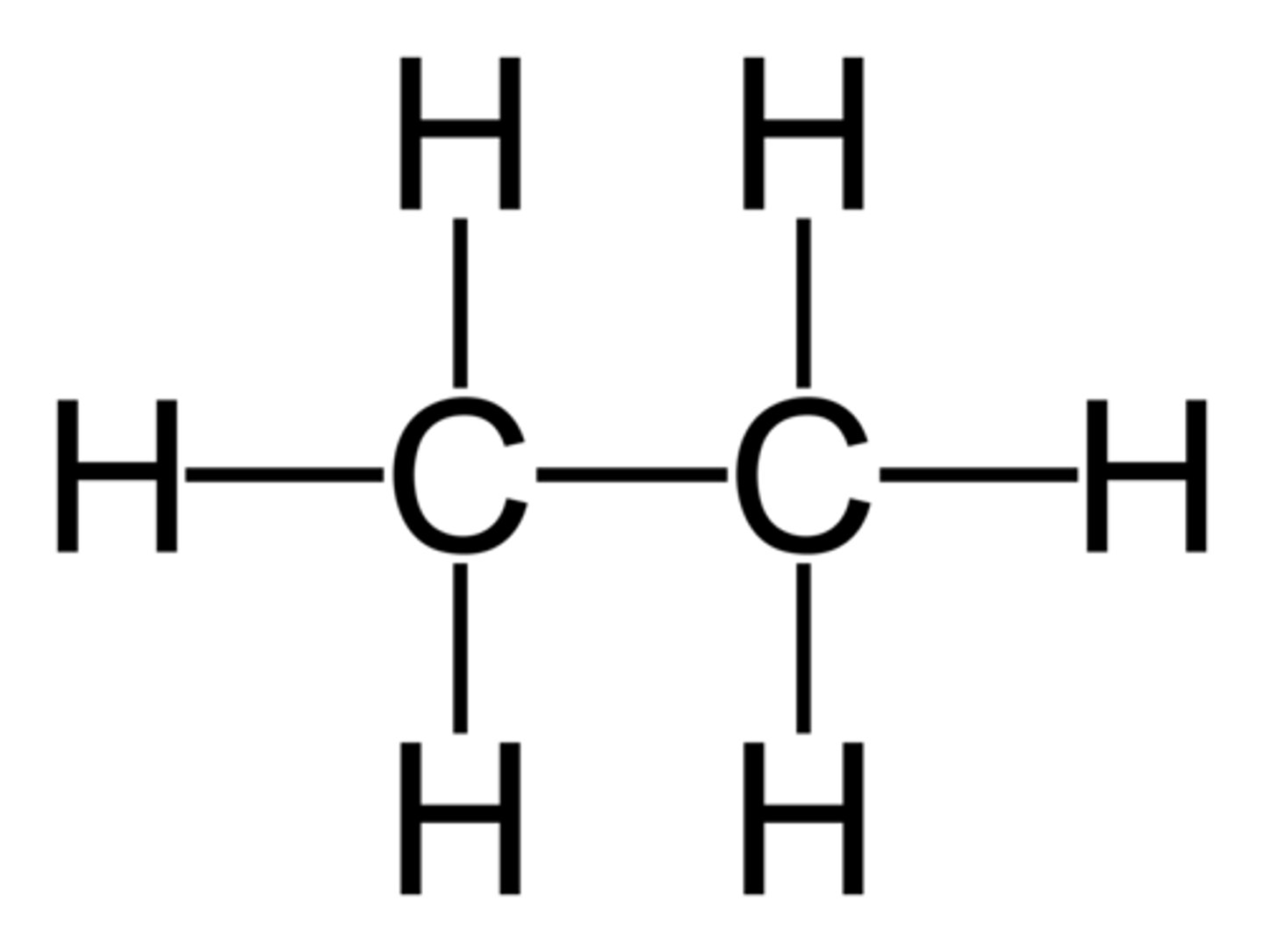
CH3CH3
Ethane structural formula
In brackets, for example: CH3CH(CH3)CH3
How are branches represented in the structural formula?
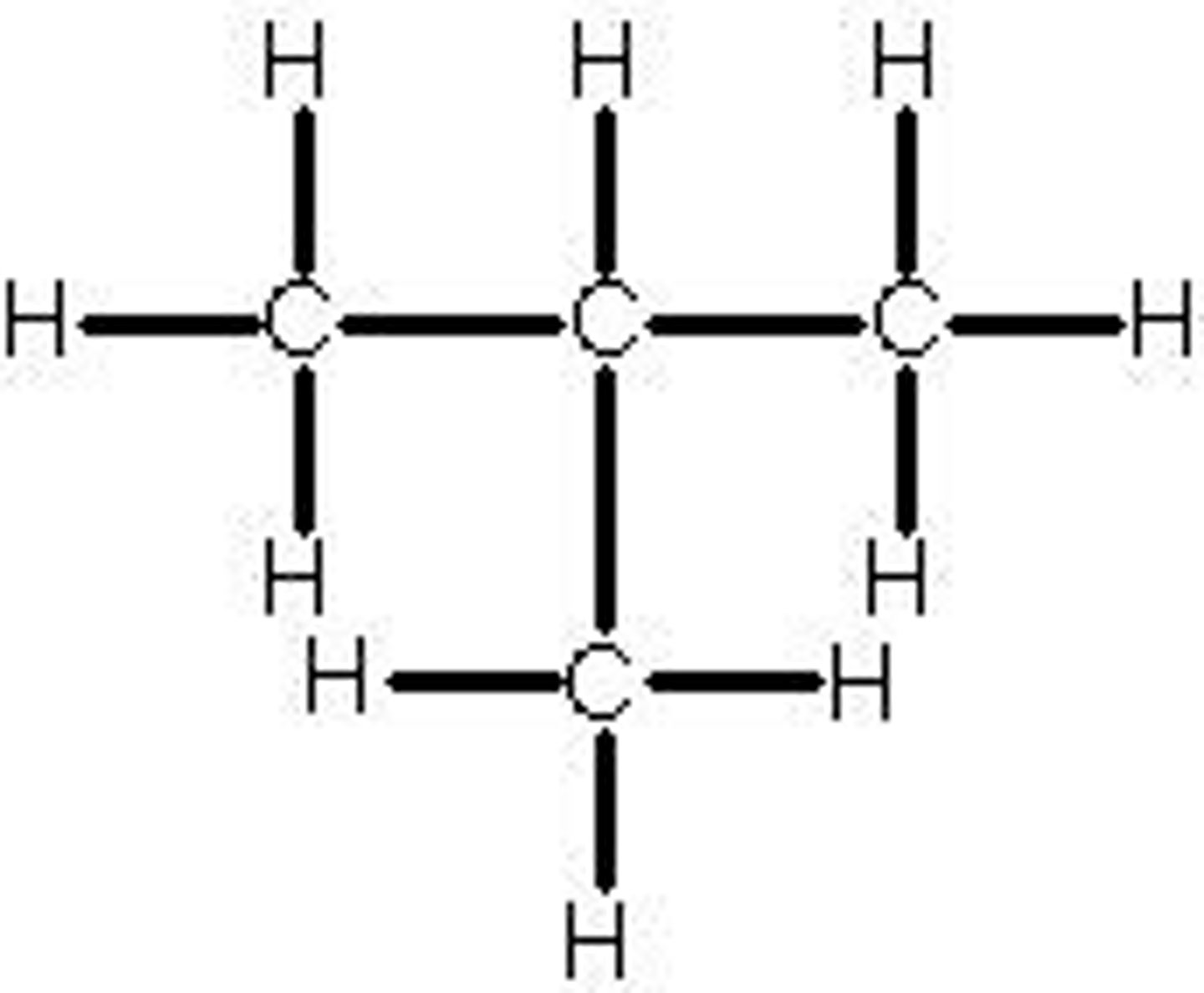
How many carbons in the longest hydrocarbon chain/ring
1 - meth
2 - eth
3 - pro
4 - but
5 - pent
6 - hex
If the root ends in "ene" there will be a double bond present
What does the root of a systematic name tell us? Identify the 6 roots
Prefixes tell us the number of carbons in a side chain
1 - methyl
2 - ethyl
3- propyl
4- butyl
Hydrocarbon rings - cyclo
For example 2-METHYLbutane
Identify the prefixes of nomenclature
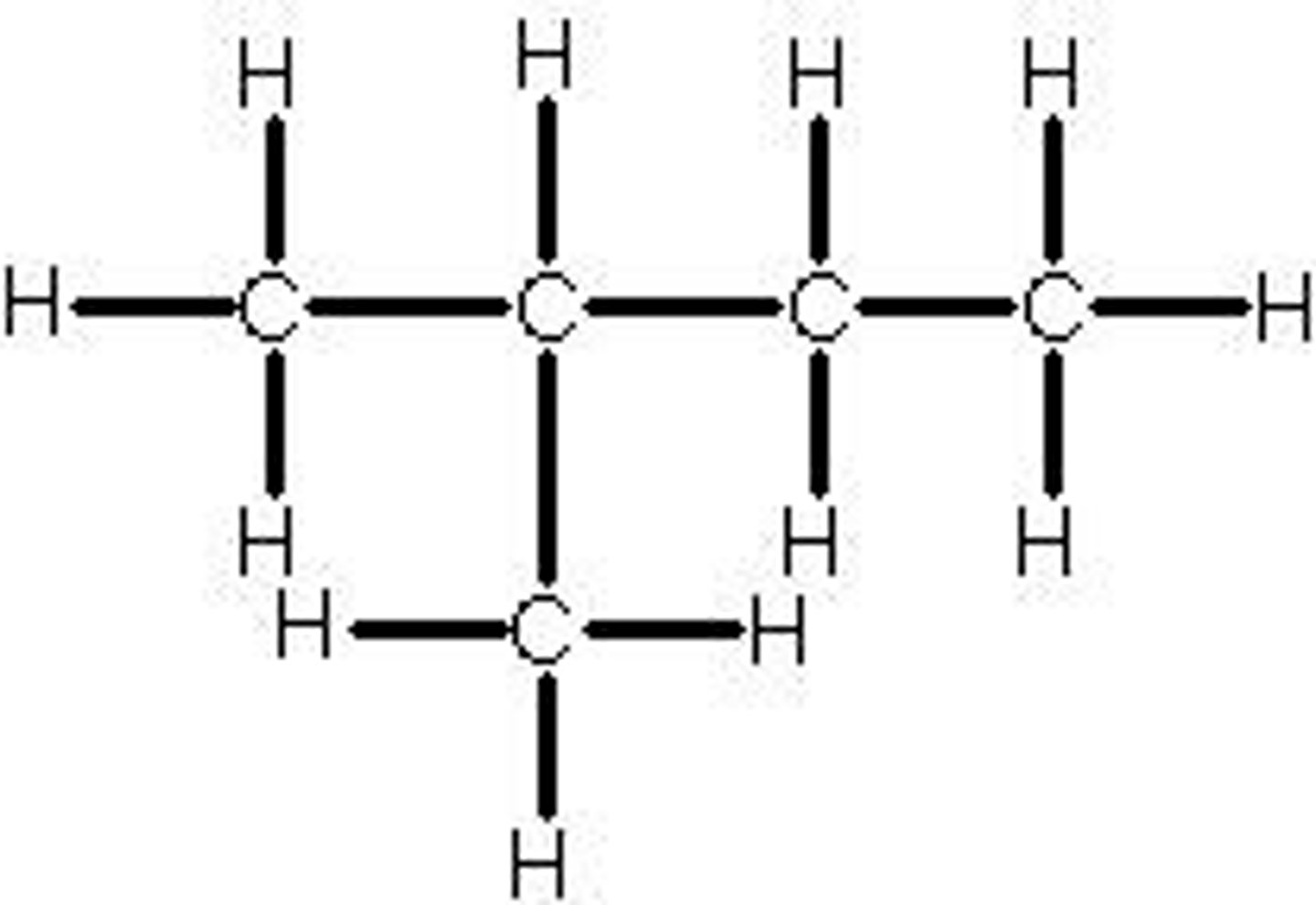
Alkane - ane
Alkene - ene
Halogenoalkane - none
Alcohols - ol
Aldehydes - al
Ketones - one
Carboxylic acids - oinc acid
Identify the suffixes of the functional groups
a) OH
b) C=OH
c) =O
d) C=O-OH
Identify the functional group for the following
a) Alcohols
b) Aldehydes
c) Ketones
d) Carboxylic acids
By a prefix
Chlorine - chloro
Bromine - bromo
Fluorine - fluoro
Iodine =- iodo
How are halogenoalkanes named?
You must number where the branch of carbons is.
In this instance the methyl group is on C2, hence
2-methyl
Displayed formula for 2-methylpentane
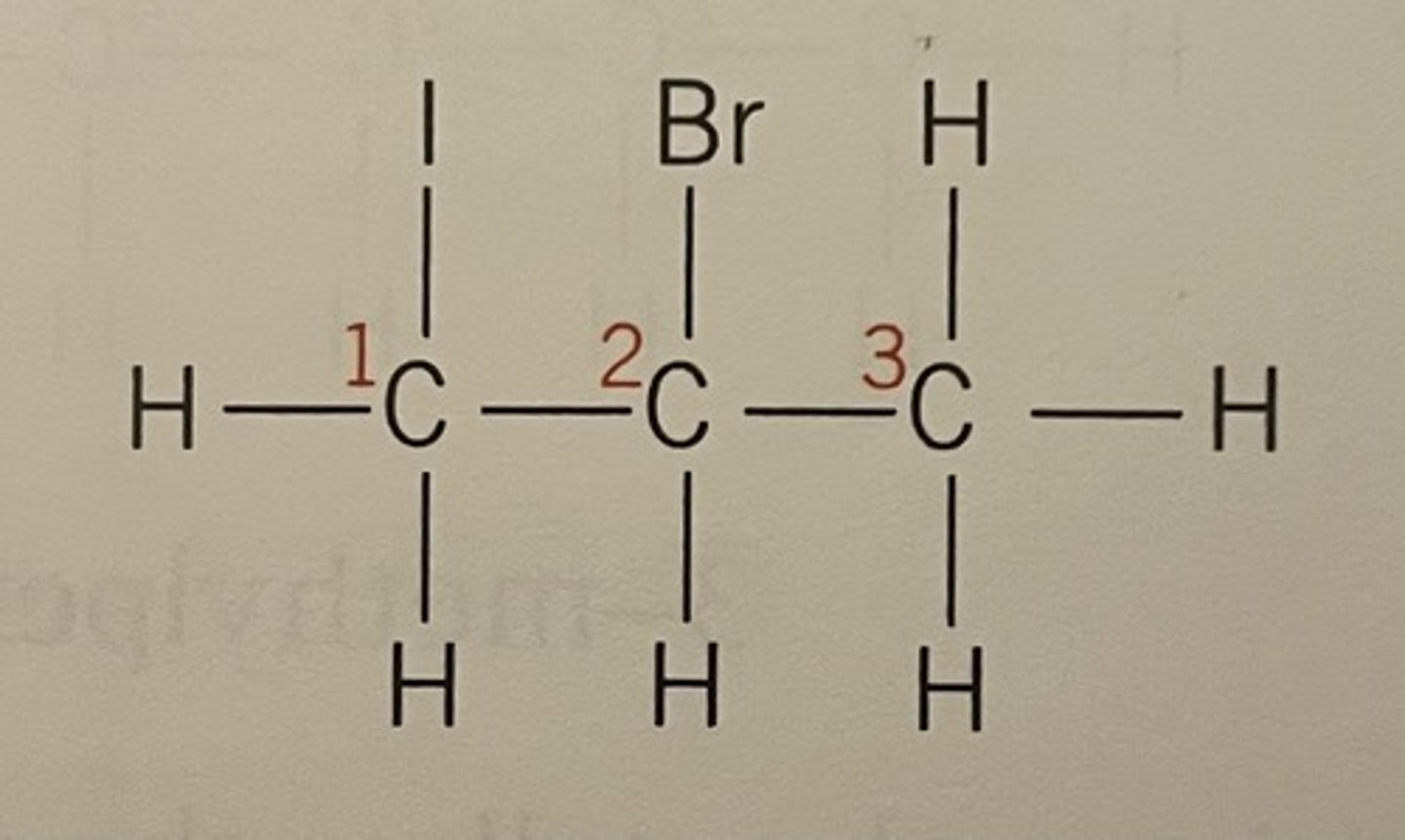
Even though iodine is on C1 and bromine is on C2, bromo comes before iodo as alphabetical order is prioritised over numerical
Displayed formula for 2-bromo-1-iodopropane
If you have more than one of the same functional group you can add either
2 - di
3 - tri
4 - tetra
In this instance both the 2 chlorine groups are on C1 hence the '1,1'
Displayed formula for 1,1-dichloroethane
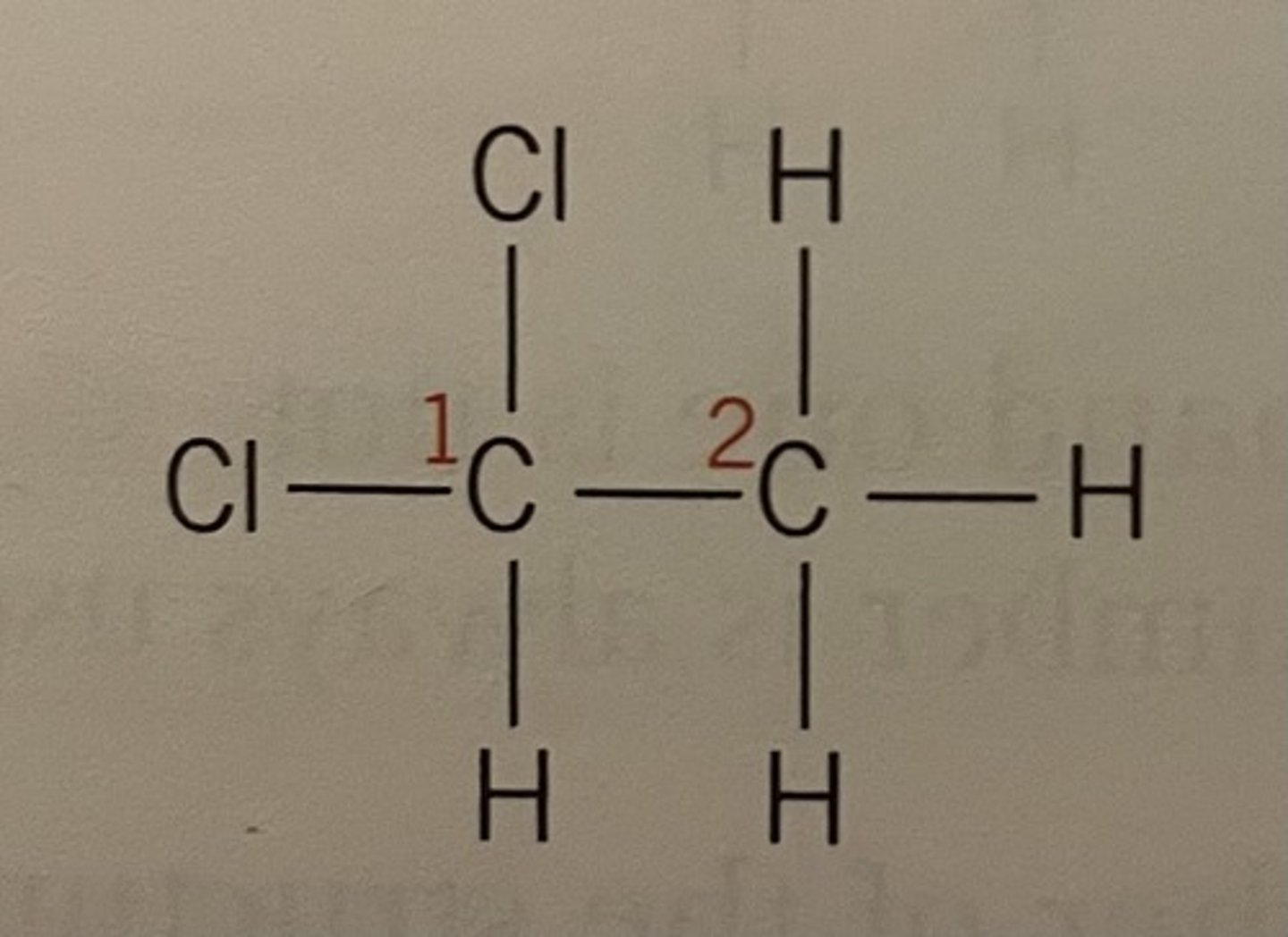
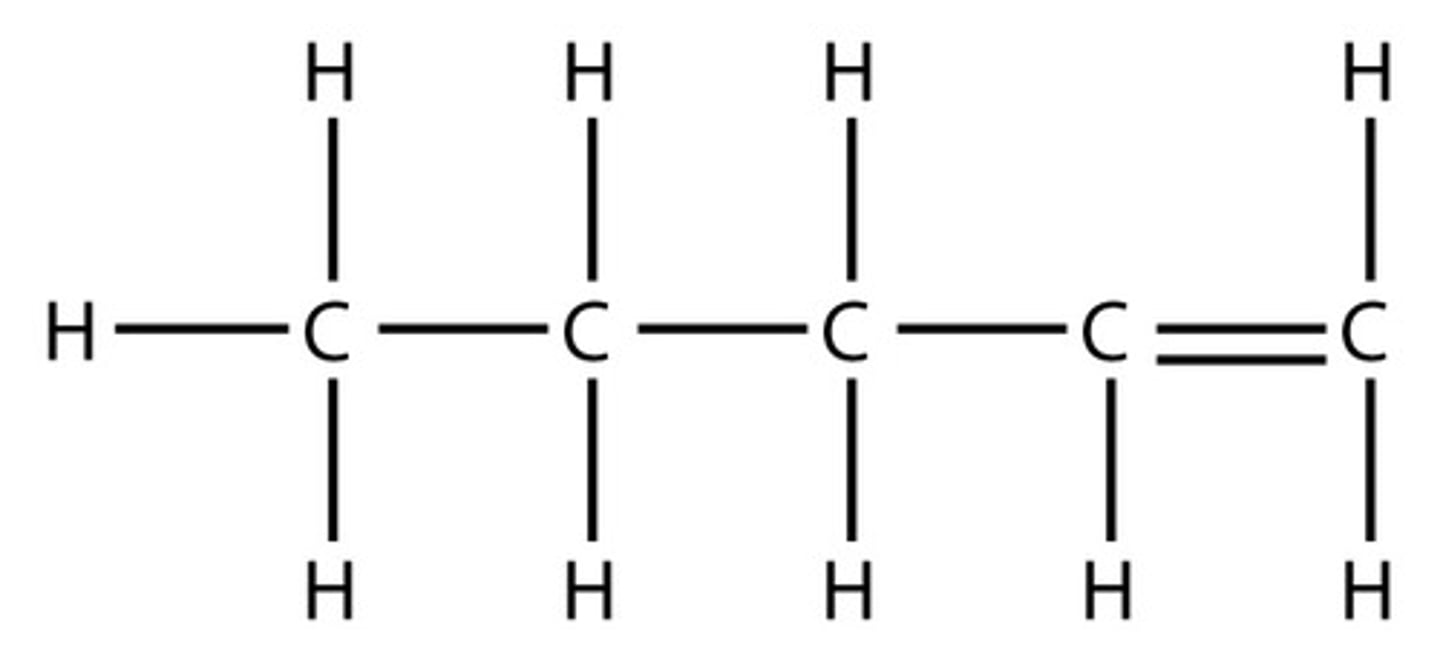
If you have a double bond, you must number where the double bond is
In this instance, we label from right to left as we need the lowest number possible
Displayed formula for but-1-ene
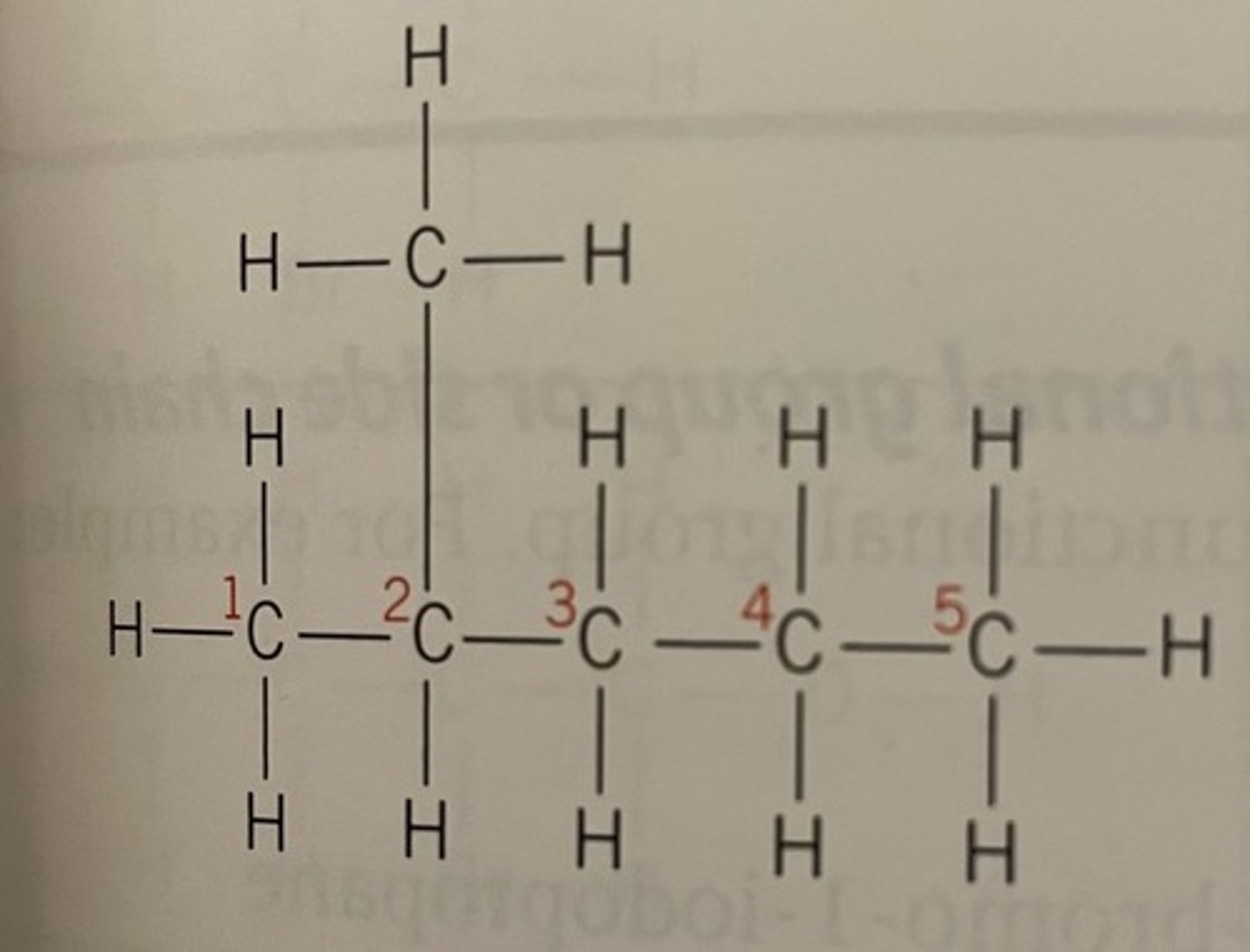
Hex - longest hydrocarbon chain is 6C long even if it bends round
Methyl group on C4
Displayed formula for 4-methylhex-1-ene
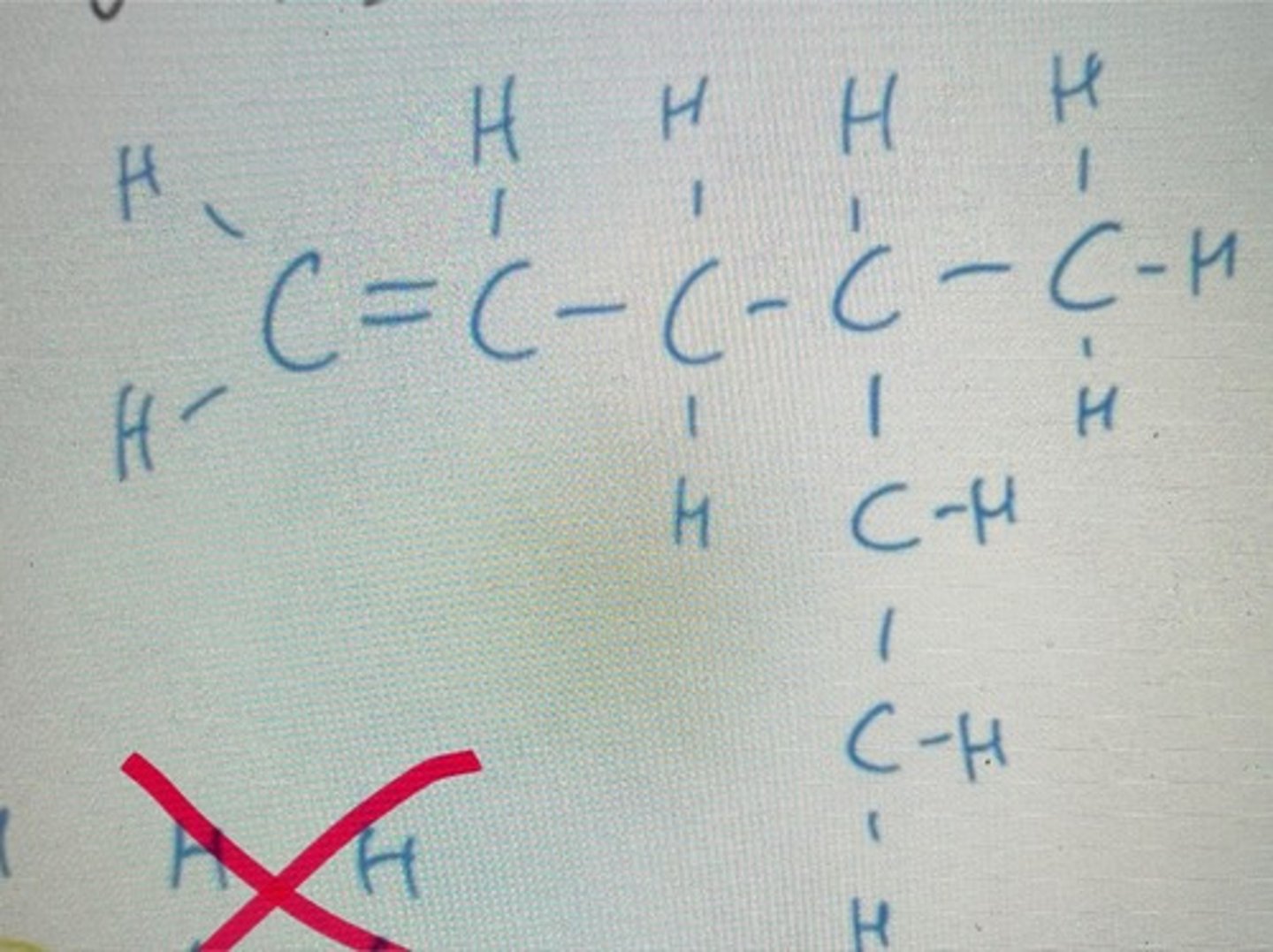
Number from right to left as this gives the lowest numbers
Displayed formula for 2,3-dimethylbut-2-ene
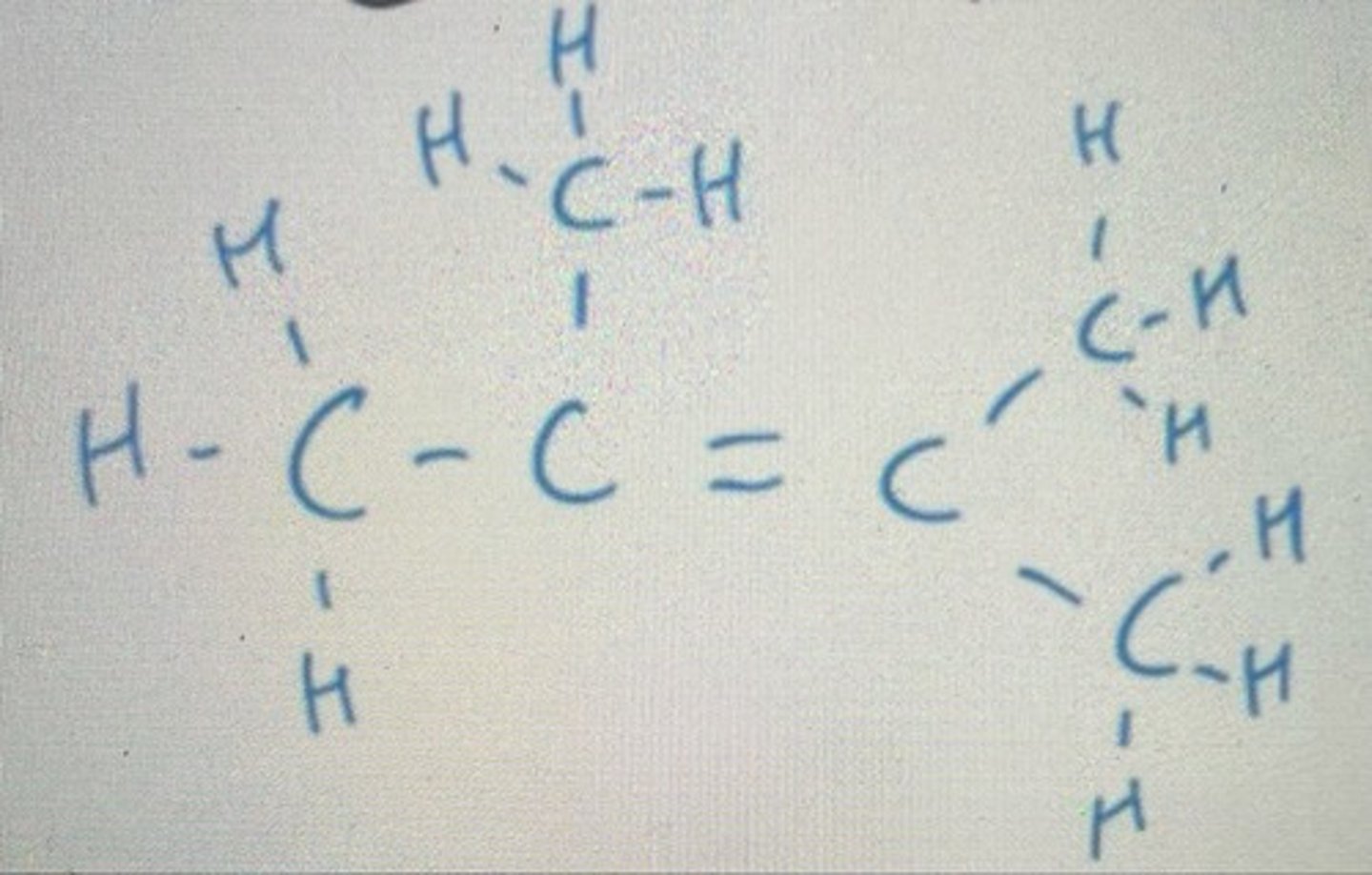
Number from right to left as F comes before I in the alphabet. We want to give the functional group with the highest precedence the lowest number
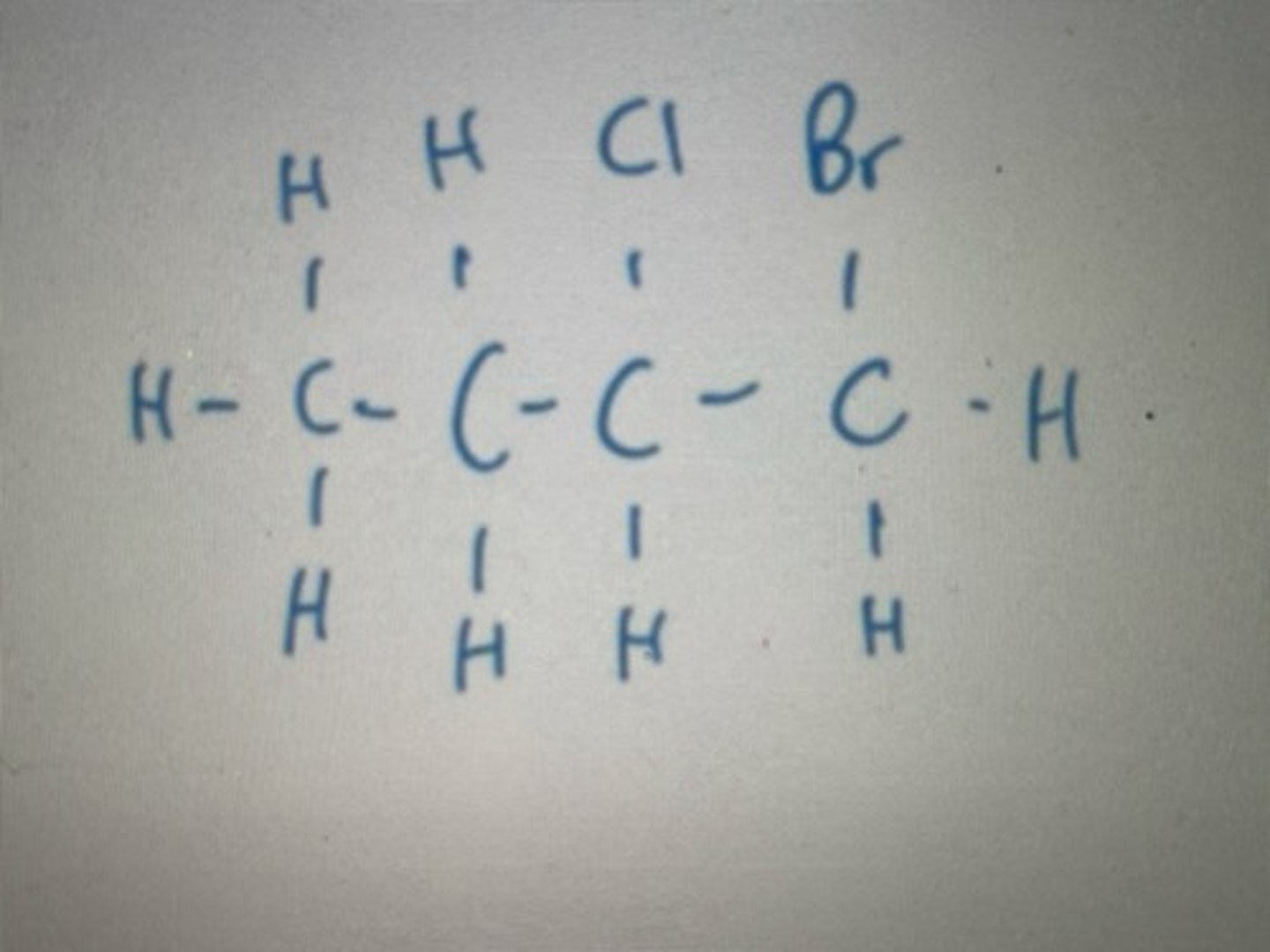
Displayed formula for 1,2-difluoro-3,4-diiodobutane
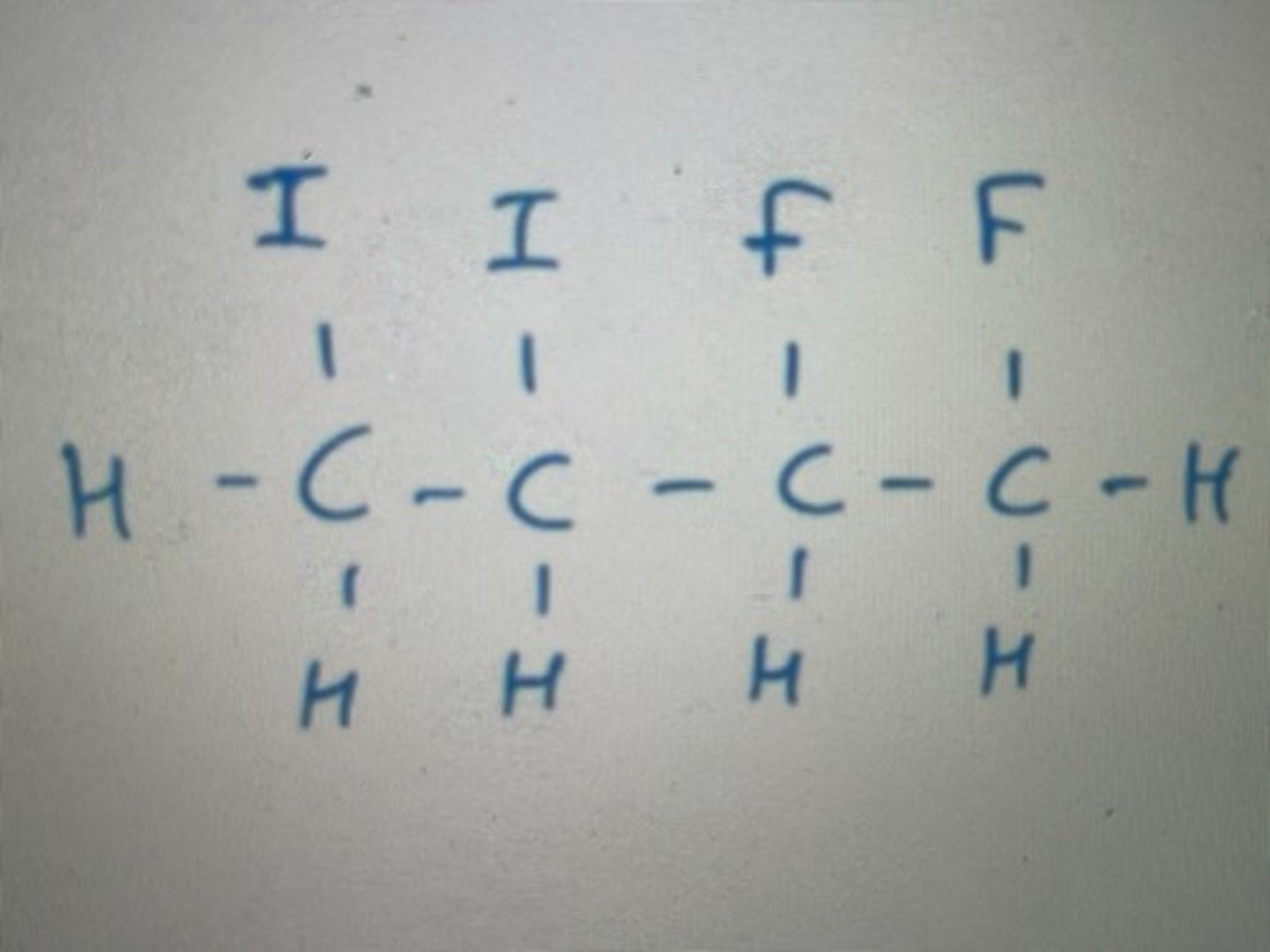
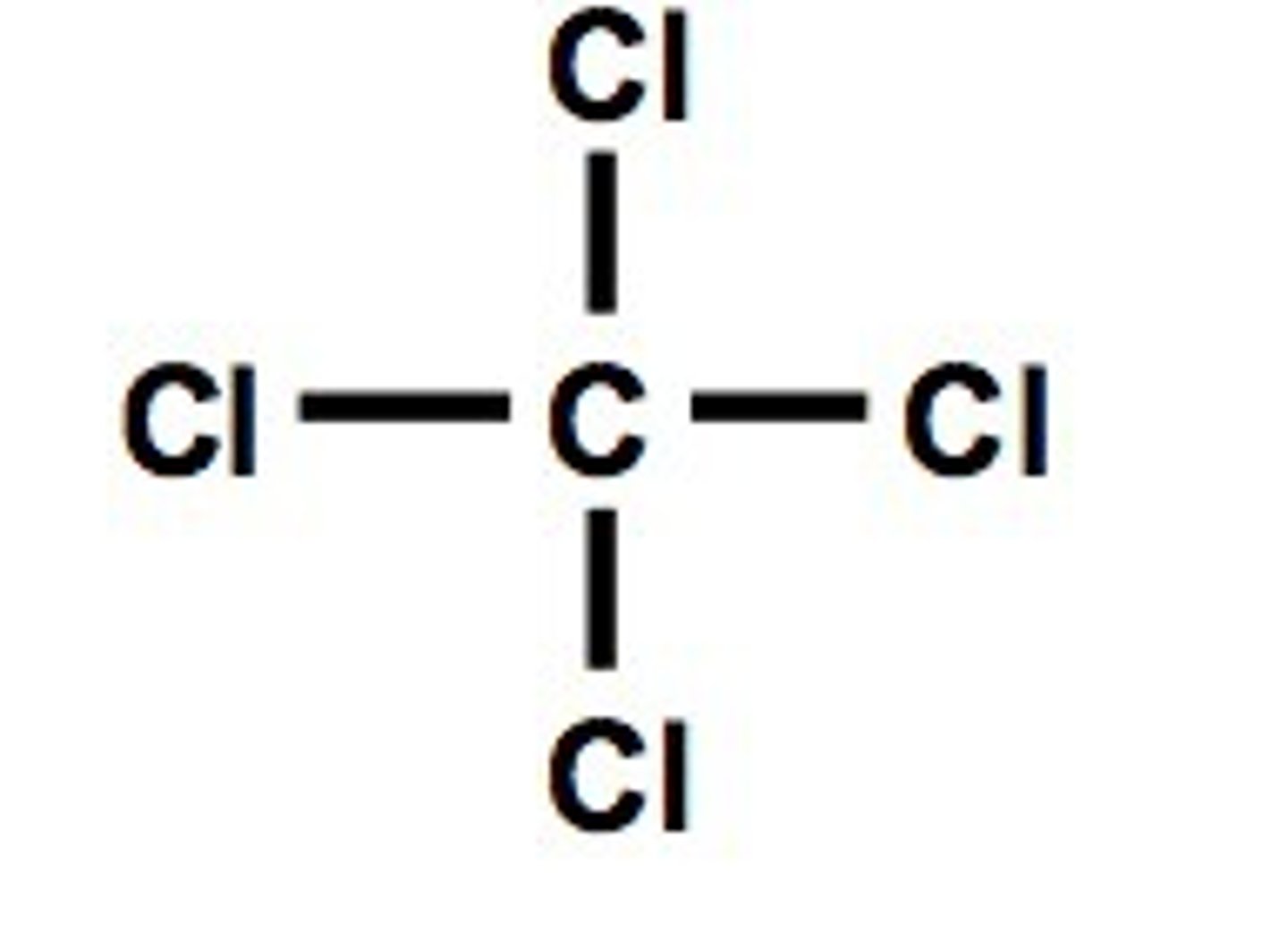
.
Displayed formula for tetrachloromethane
.
Displayed formula for 1-bromo-2-chlorobutane
.
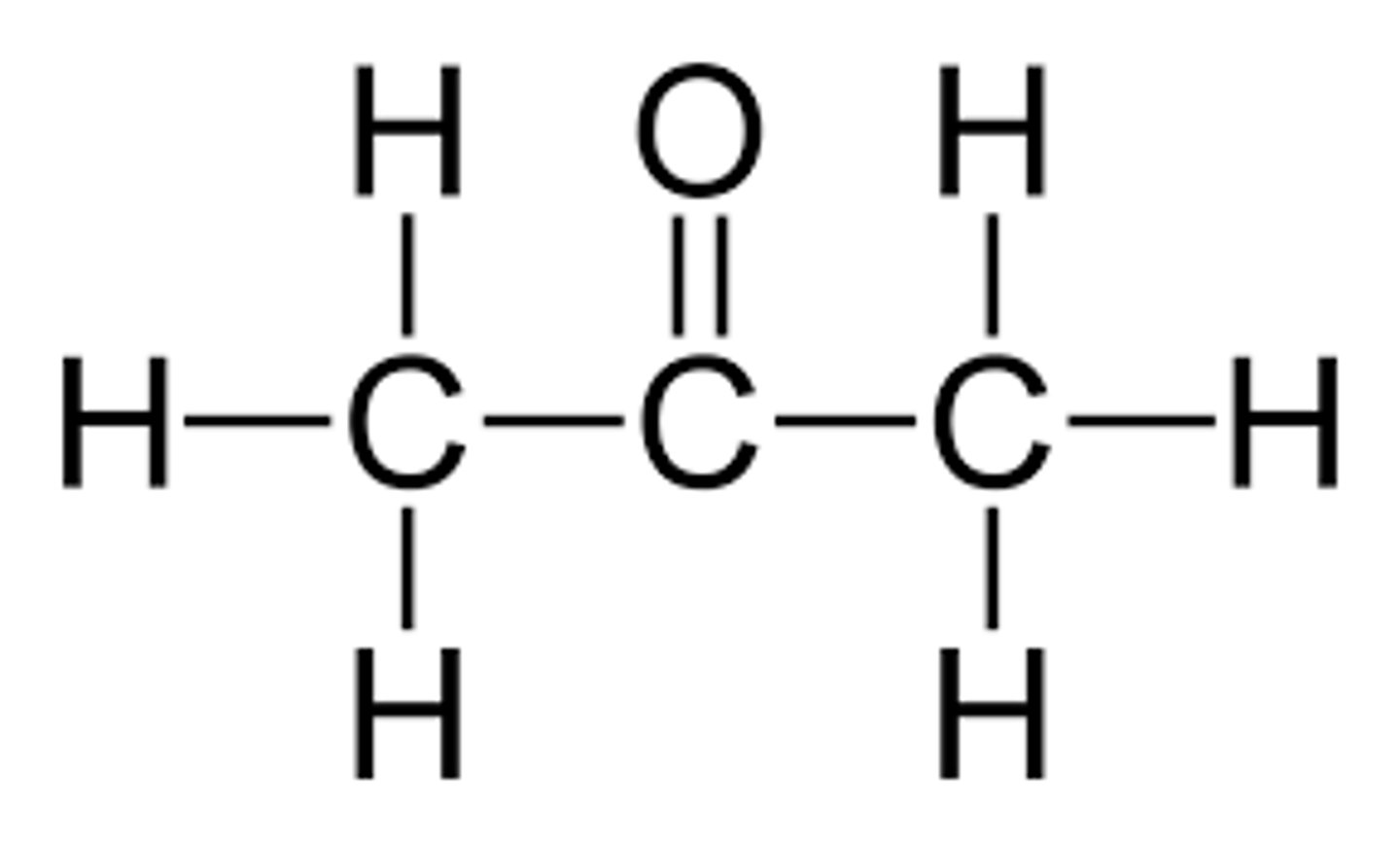
Displayed formula for propanone
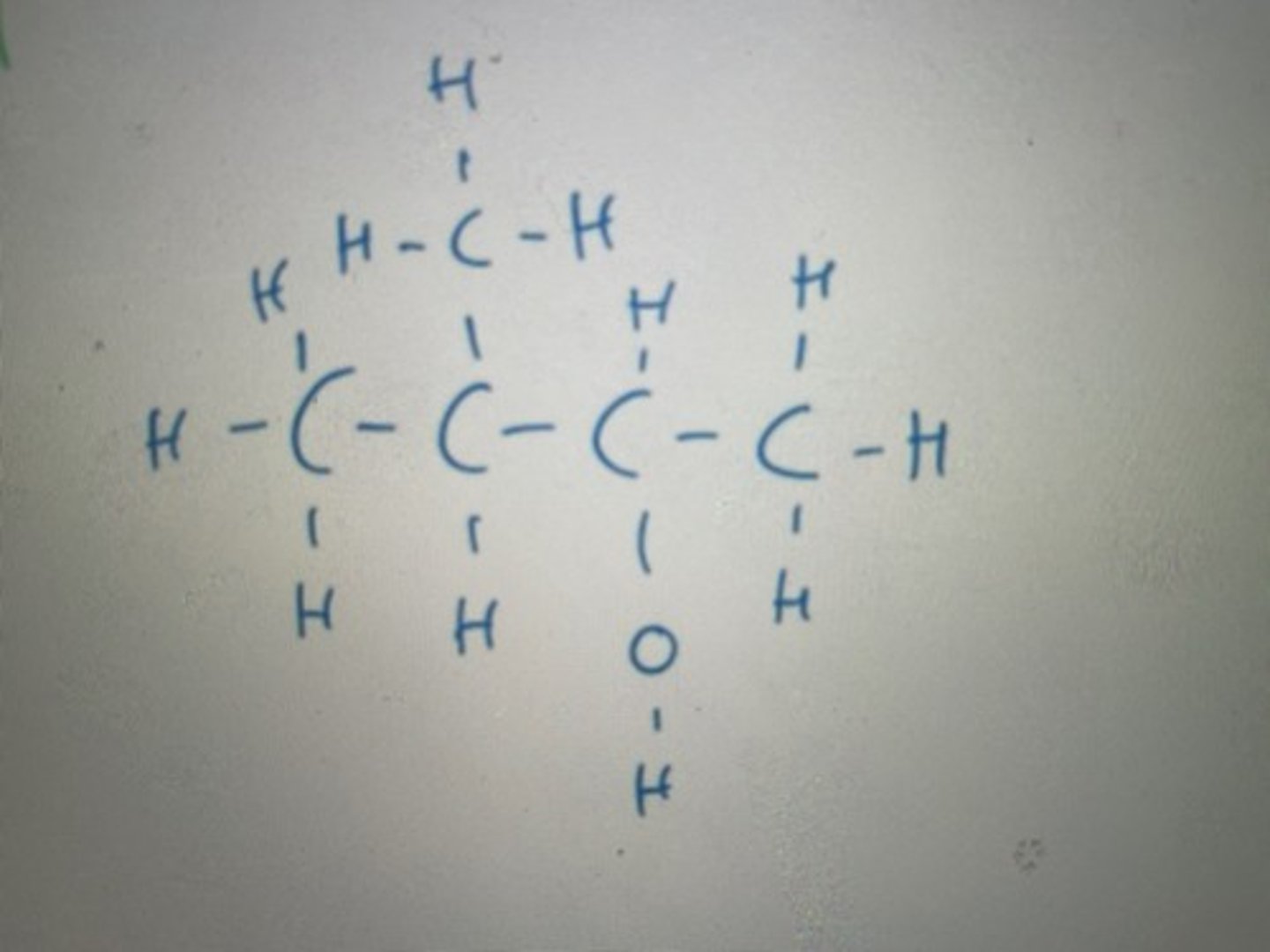
Number from right to left as the OH functional group takes priority over methyl chain
Displayed formula for 3-methylbutan-2-ol
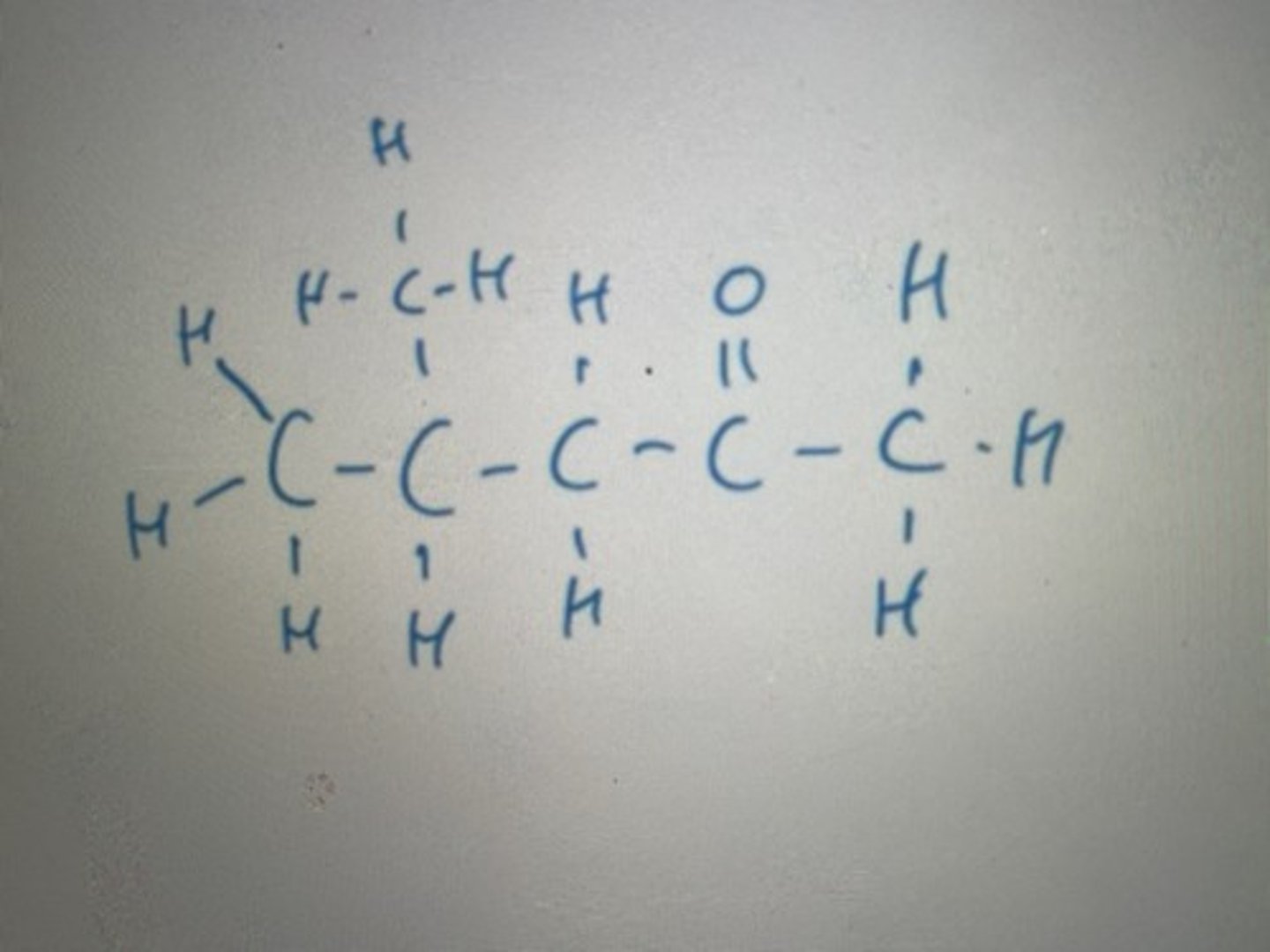
.
Displayed formula for 4-methylpentan-2-one
"al" ending as the functional group C=O is aldehyde
I
H
Displayed formula 2-methylbutanal
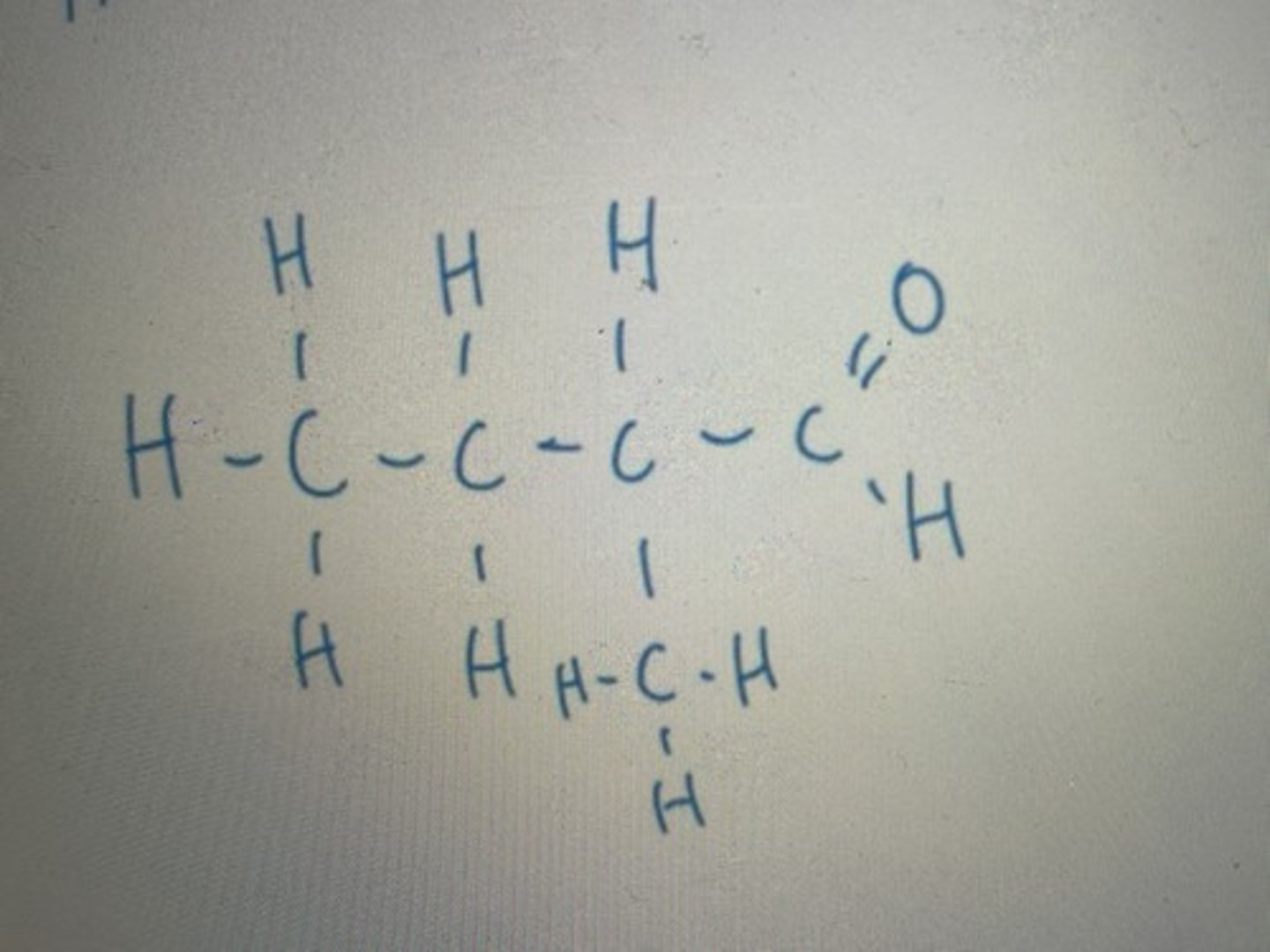
"one" ending as the functional group group is =O (ketone)
Number from right to left as function group takes priority
Displayed formula for 4-methylpentan-2-one
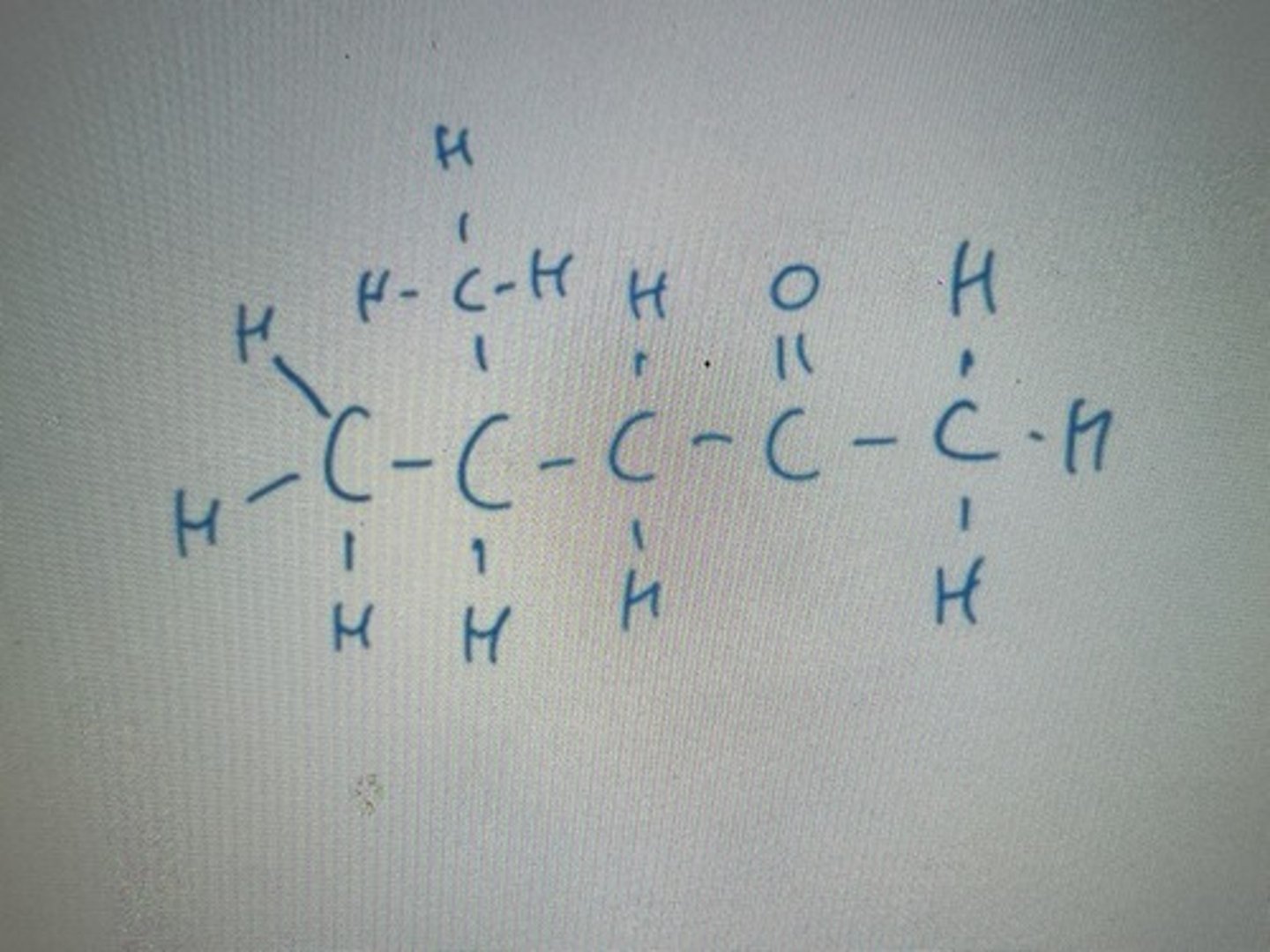
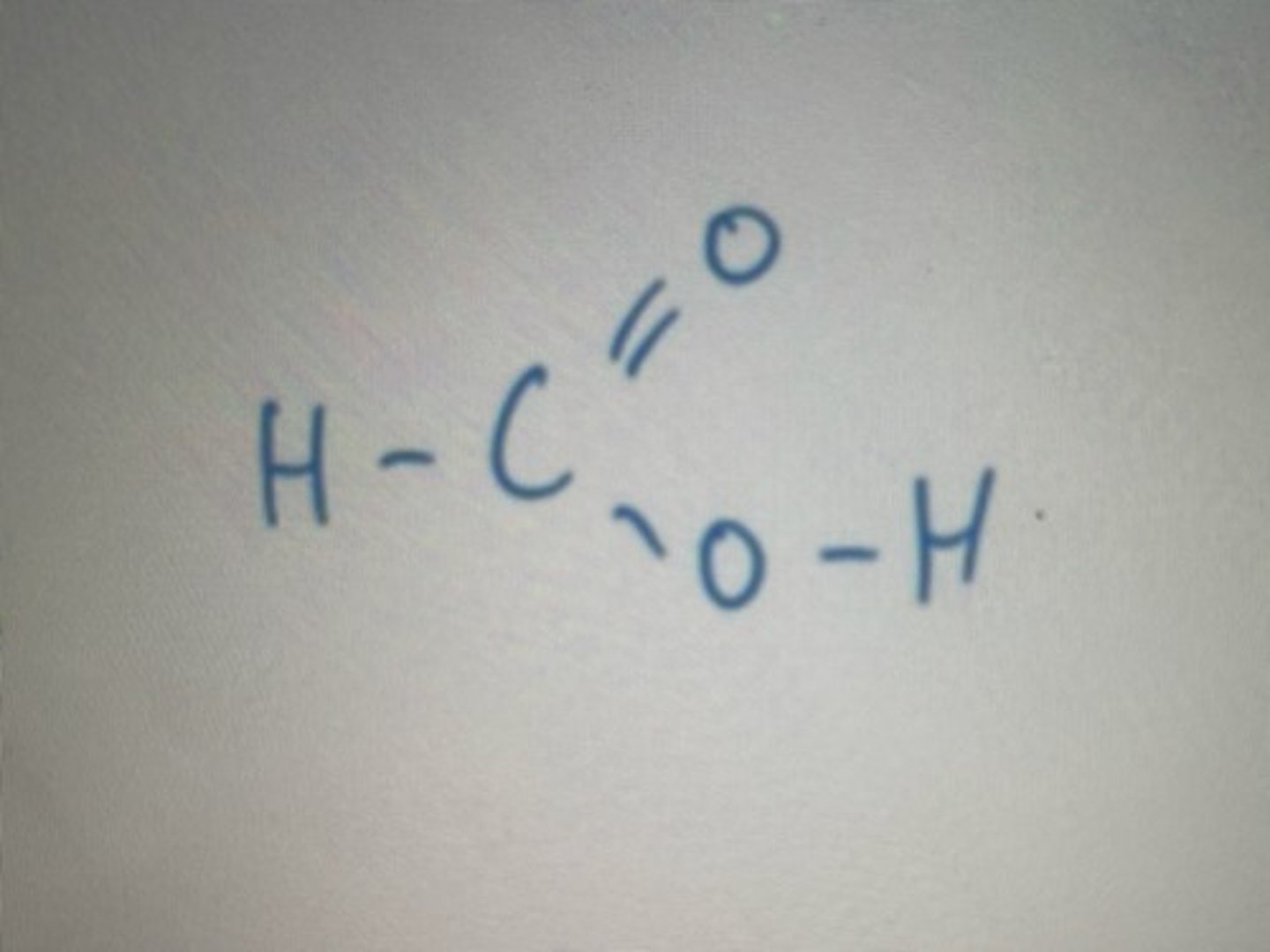
COOH carboxylic functional group gives "oic" acid suffix
Displayed formula for methanoicacid
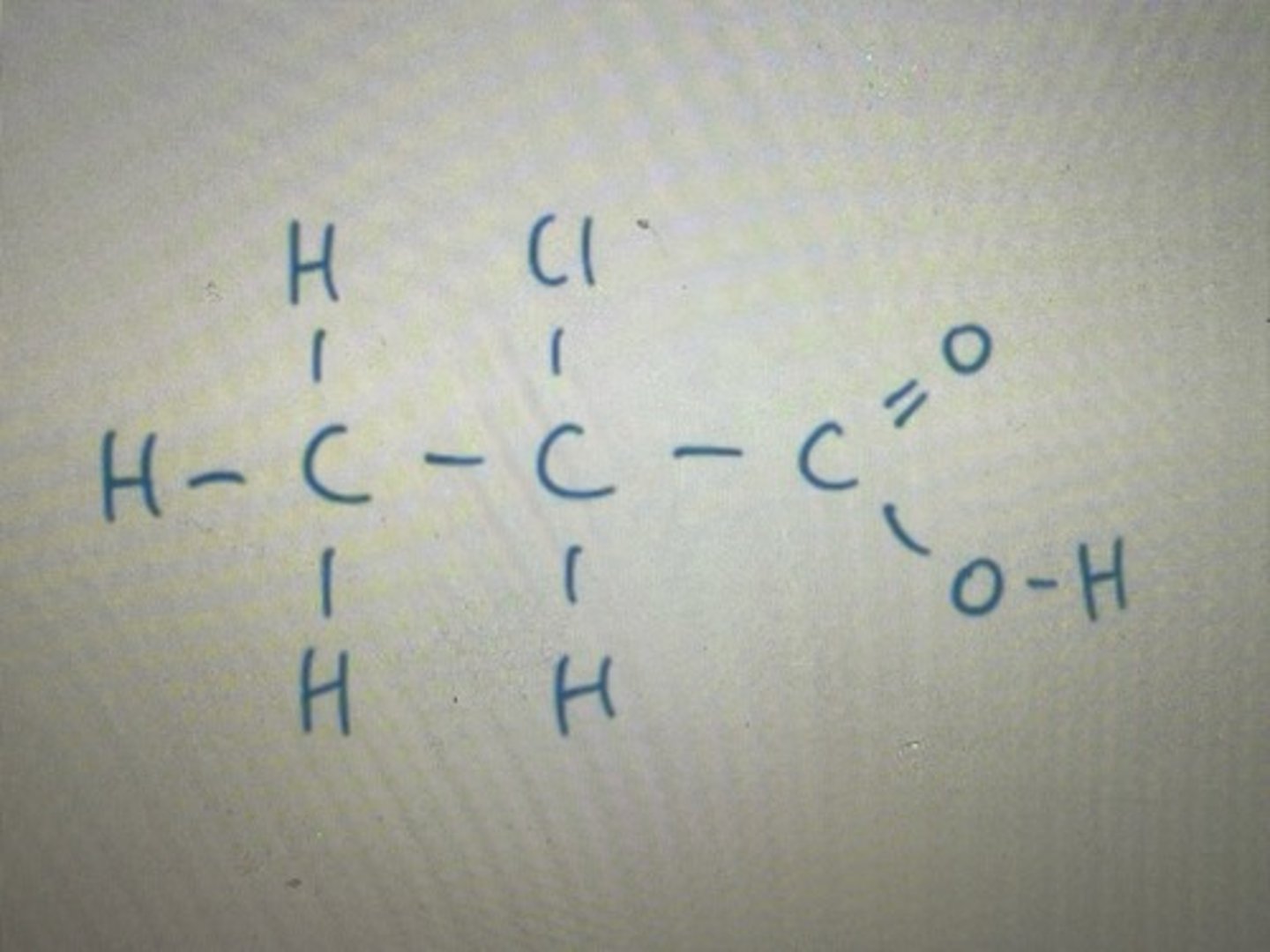
Carboxylic acid group always on the end no need to number
Displayed formula for 2-chloropropanoicacid
Compounds with the same molecular formula but different structural formula
Define structural isomer
a) different arrangement of hydrocarbon chain (such as branching)
b) same functional groups attached to the main chain at different points
c) different functional groups
What is
a) Chain isomerism
b) Positional isomerism
c) Functional isomerism
2-bromo-propane
Both have molecular formula CH3CH7BR
Same function group Bromine but on different Carbons
Positional isomer of 1-bromo-propane
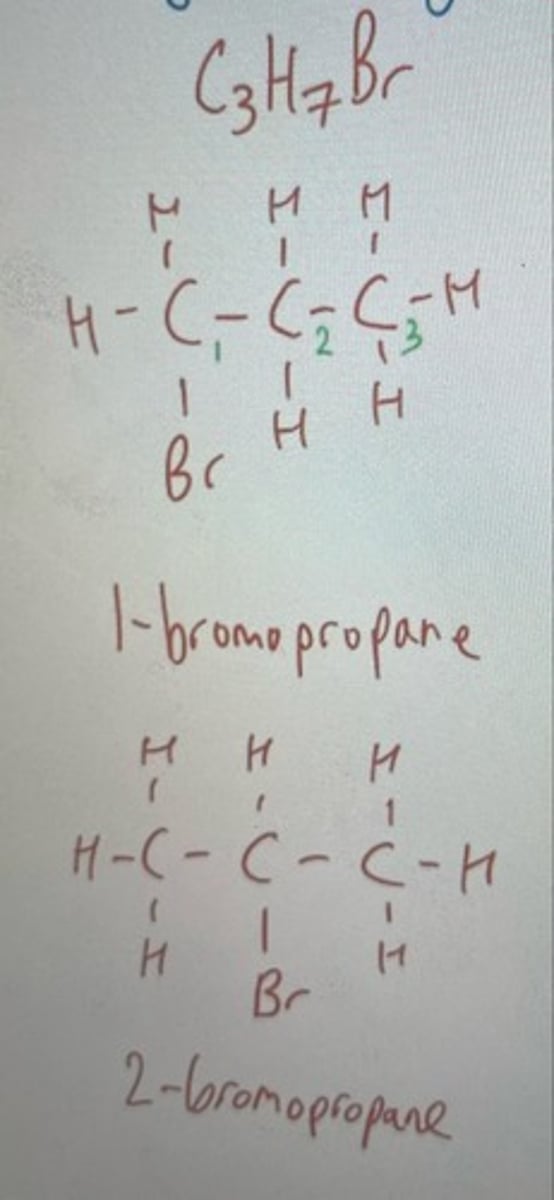
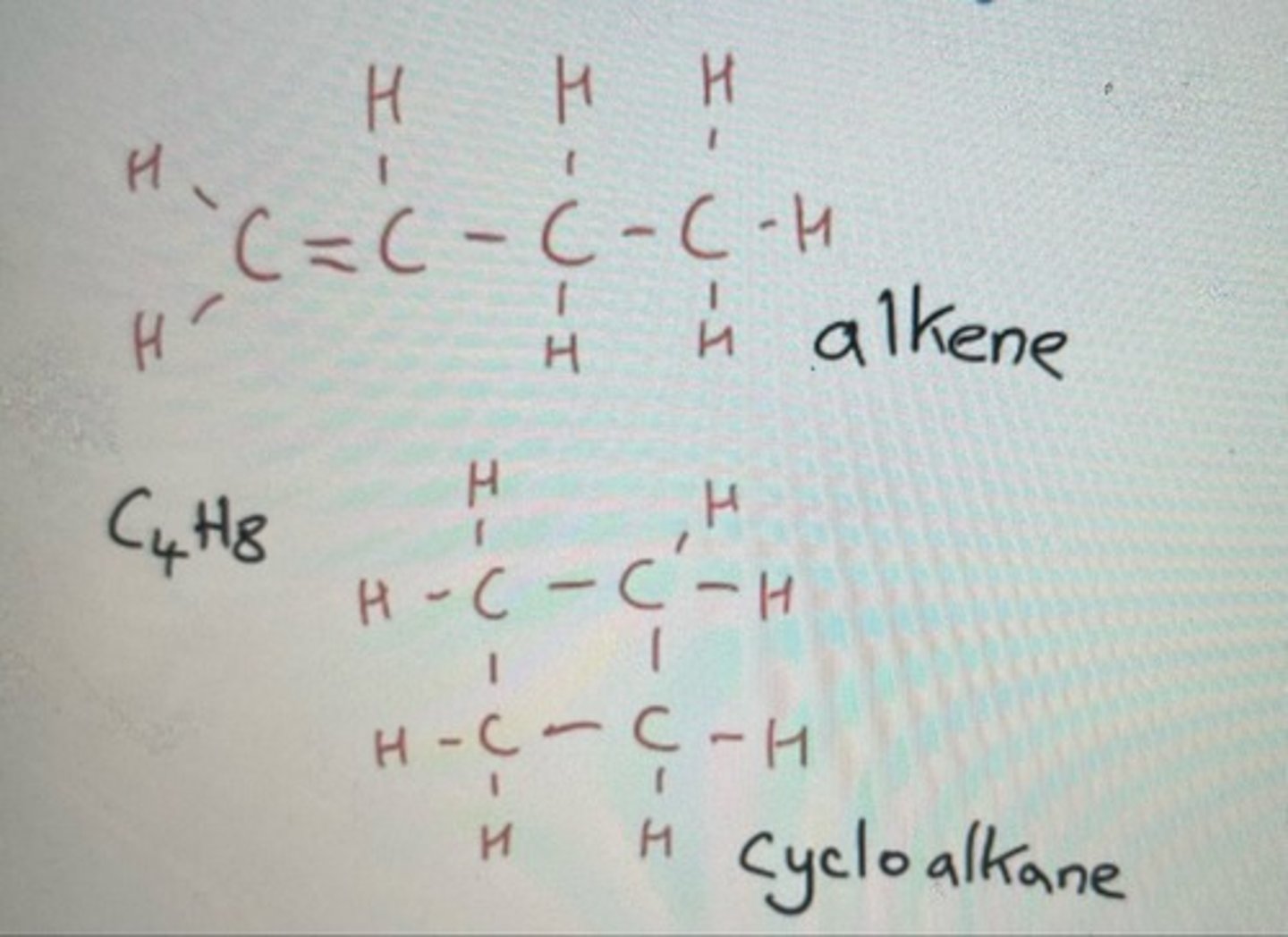
Same molecular formula but the double bond has been removed (functional group has changed)
Alkenes pair with cycloalkanes when producing functional group isomers
Functional group isomer of C4H8
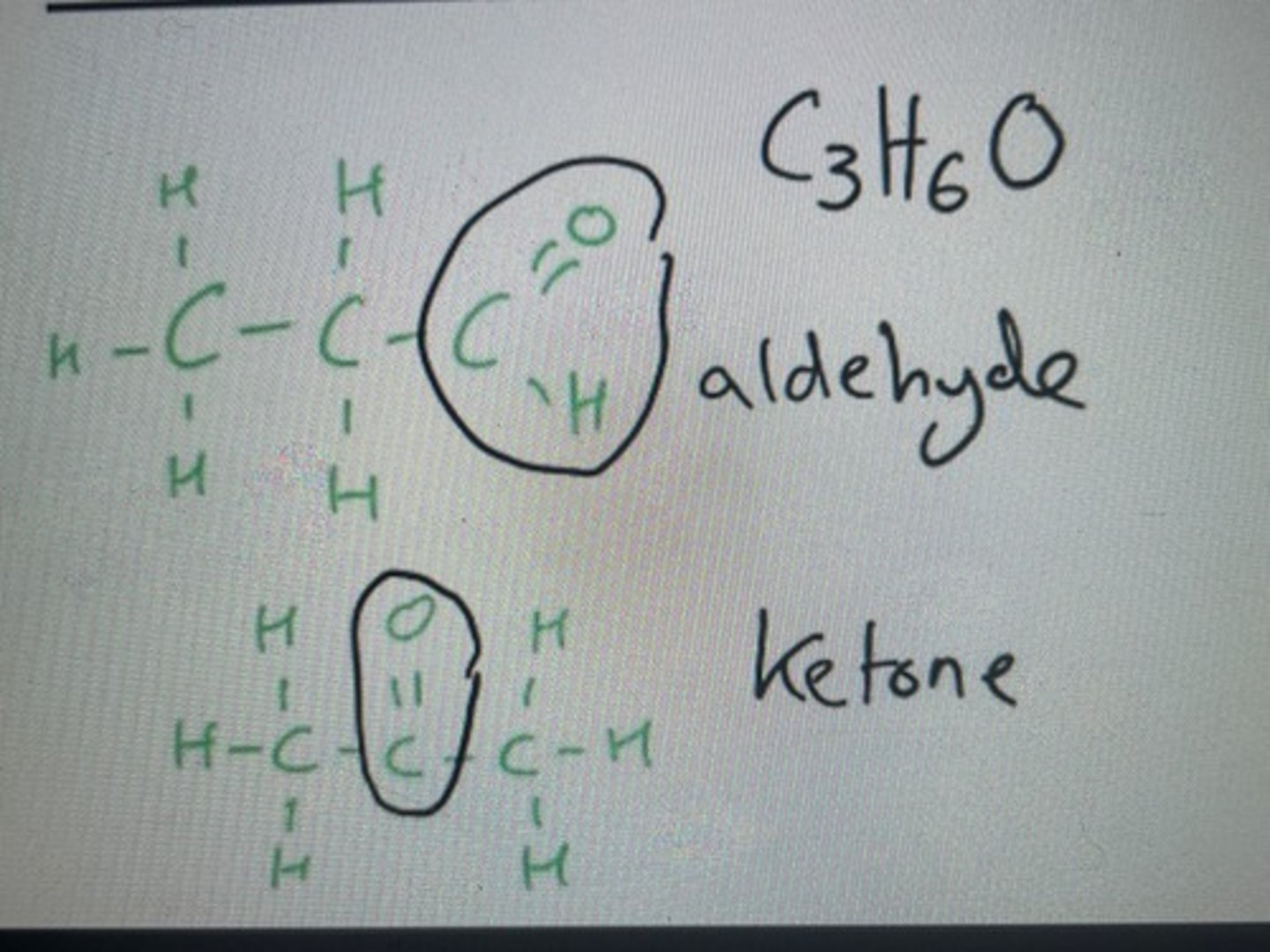
Same molecular formula but they have different functional groups (aldehyde v ketone)
Aldehydes pair with ketones when forming functional group isomers
Functional group isomer of C3H6O
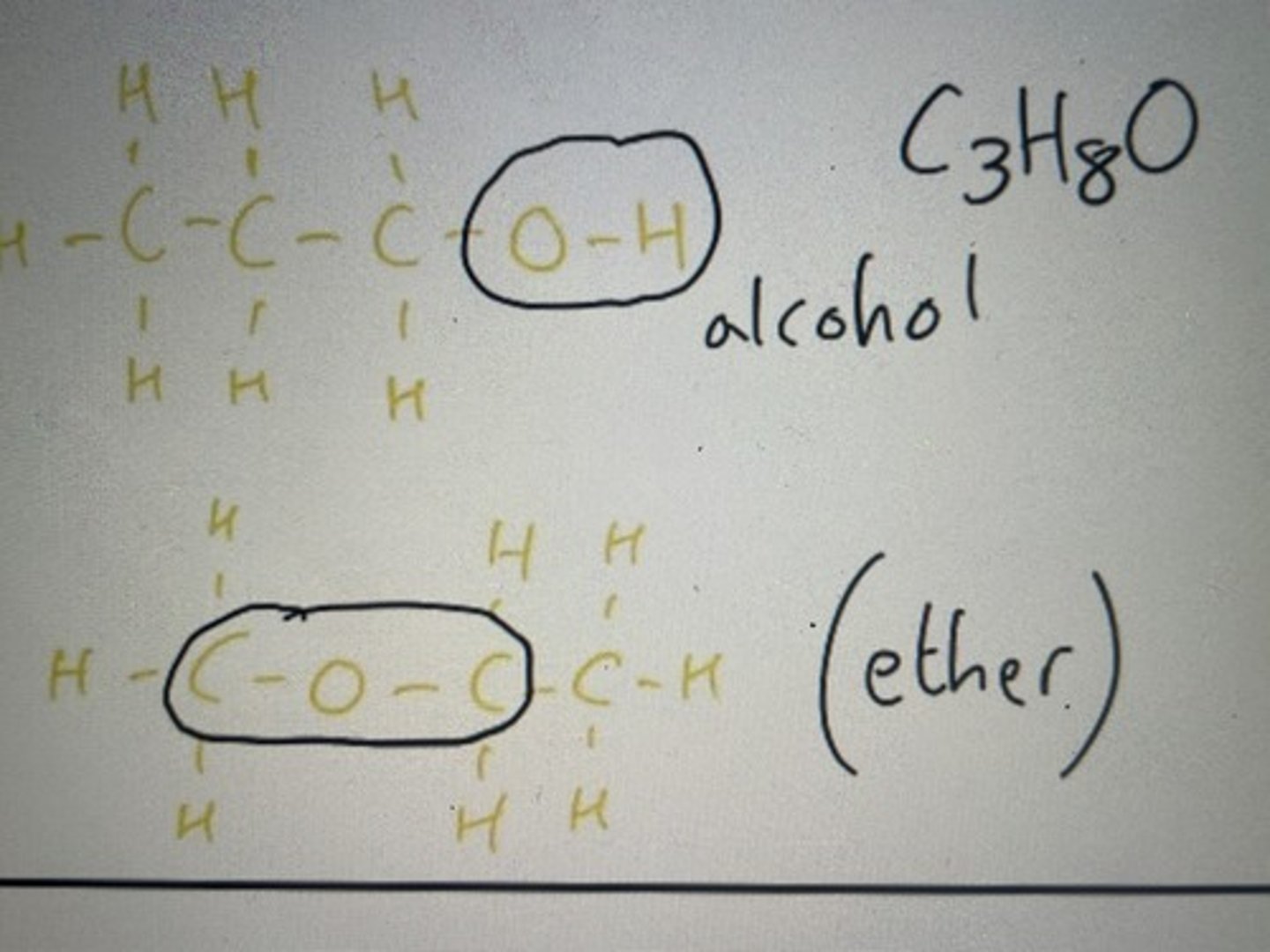
Same molecular formula but the O bonded to the H creates an alcohol group, whereas the other O is simply bonded to carbon atoms (ether)
Alcohols form pairs with ethers when producing functional group isomers
Function group isomer of C3H8O
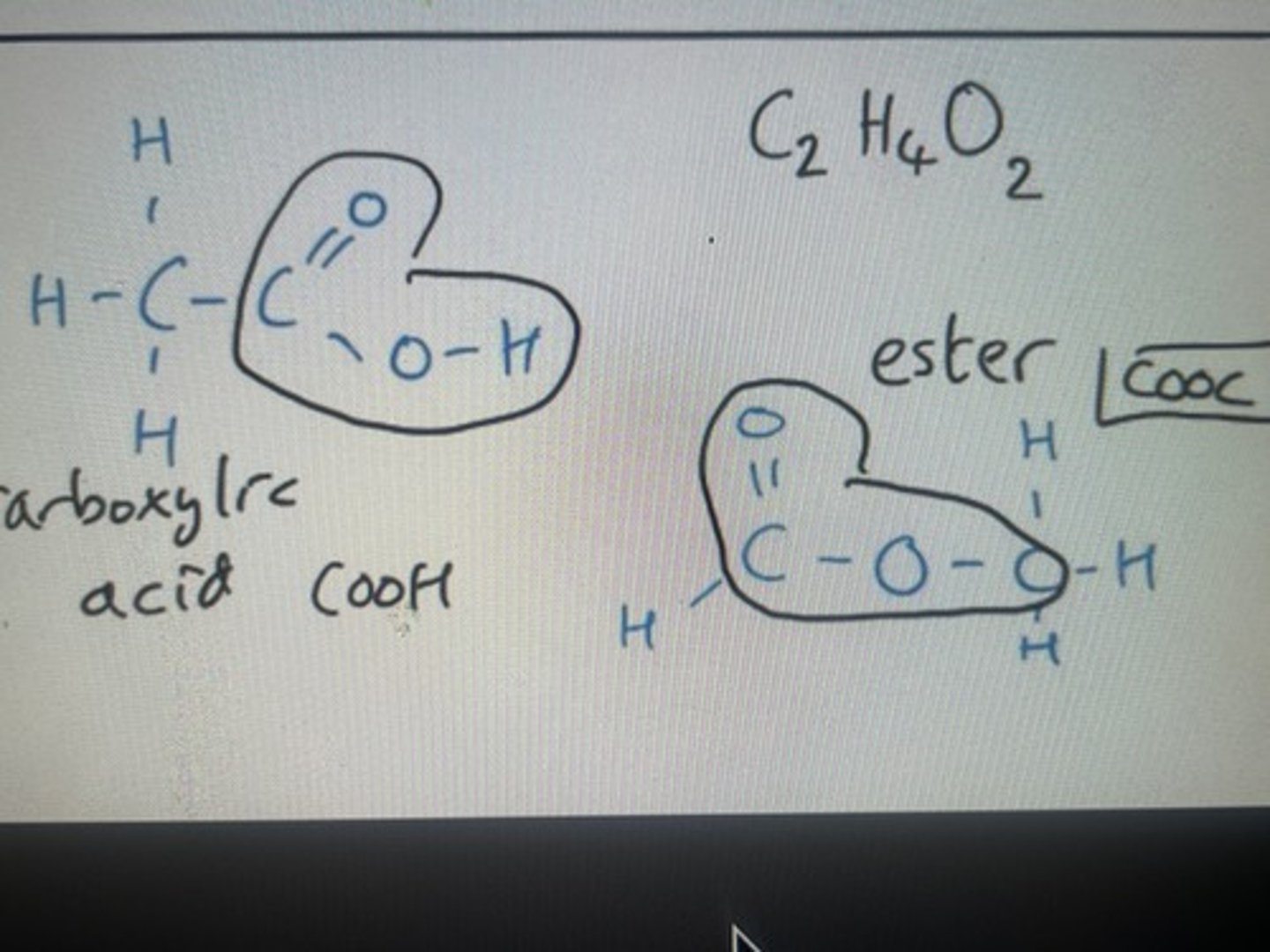
Same molecular formula but functional group as changed from COOH (carboxylic acid) to COO (ester)
Carboxylic acids and esters form pairs when producing functional group isomers
Functional group isomer of C2H4O2
4
How many structural isomers does C4H9Br have?

5
There is no function group, it is just an alkane, this means it can only have different types of CHAIN isomers.
Make sure you don't just create a chain that bends round
How many structural isomers does C6H14 have?

.
Skeletal formula for 2,2-dimethylbutane

10
How many structural isomers for C5H10 have?
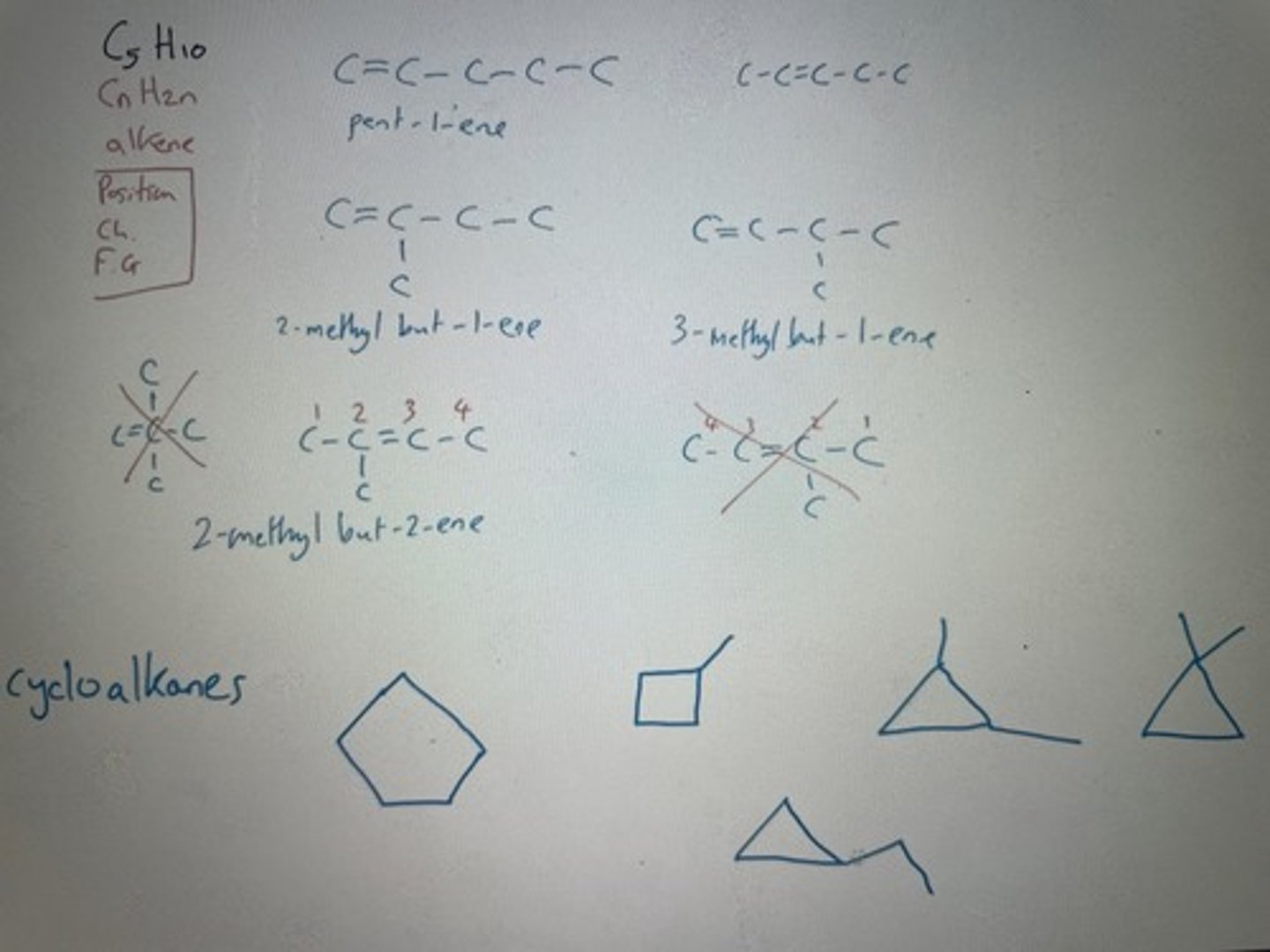
Give the IUPAC name of the chain isomer for but-1-ene
Methylpropene
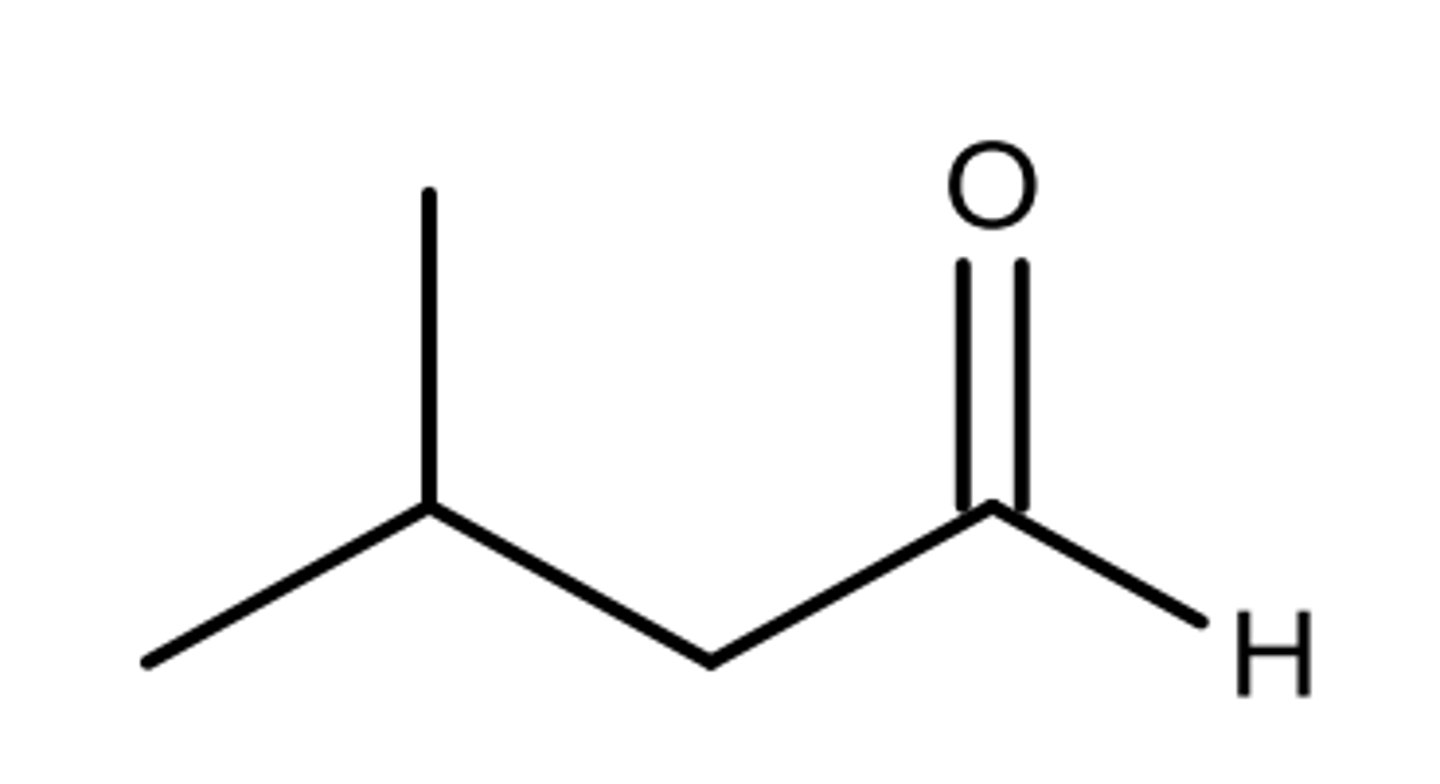
Skeletal formula for 3-methylbutanal
Draw the displayed formula of C5H11Br that is the major product of the reaction of 2-methyl-but-2-ene with hydrogen bromide
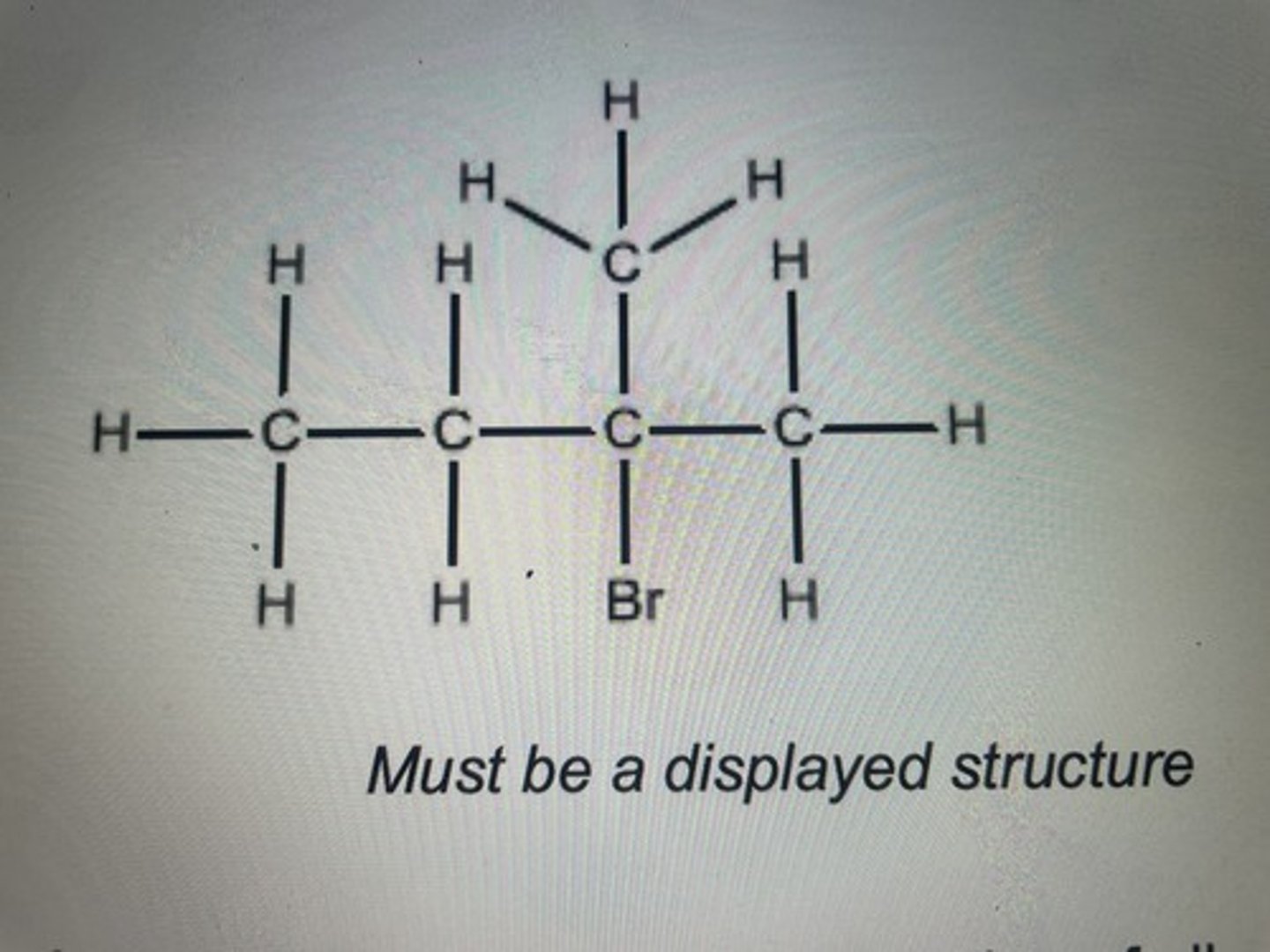
What type of structural isomers can alkanes form
Chain only
How many structural isomers does C6H14 have
5
What is stereoisomerism?
Same structural formula but different arrangement of atoms in space
What is E and Z isomerism?
E = Priority group (highest atomic number) on diagonal side
Z = Priority group (highest atomic number) on same side
Compare either side of the double bond highest atomic number to find the priority group
But-2-ene E and Z isomerism
1,2-dibromo-1iodoethene E and Z isomerism
2,3-dihydroxylbut-2-ene E and Z isomerism
2-chloro-1-iodopropene E and Z isomerism
Draw the structure of (Z)-pent-3-en-2-ol