Topic 4: Basics of Cell Energy
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
How do organisms transform energy?
Absorb energy (light or chemical) → Releases energy (thermal energy + metabolic wastes)
How are organisms governed by the law of thermodynamics?
▪ Energy is neither created nor destroyed, only transferred and
transformed
▪ Every energy transfer or transformation increases the entropy of
the surroundings
What happens when some energy in any transformation from aa cell becomes heat
→ Heat = most disorganized form of energy, therefore release of heat = increase in entropy
→ Not available to do work, and is lost from system
What happens if an energy transformation results in an increase in entropy
Chemical reactions will proceed spontaneously (without adding energy)
Can a non-spontaneous process happen in the cell?
Yes! But… energy has to be added
Creates localized area of lowered entropy
Exergonic Reaction
Releases free energy (ΔG less than o)
Occurs spontaneously
Ex: Breaking polymers into monomers
Endergonic Reaction
Absorbs free energy from the surroundings to power the reaction (ΔG greater than 0)
Not spontaneous
Ex: Making a polymer from monomers
Metabolism
All of an organism’s chemical reactions
Organized into pathways
Metabolic Pathway
The chemical reactions from a starting material to an ending material
Each step catalyzed by a different enzyme
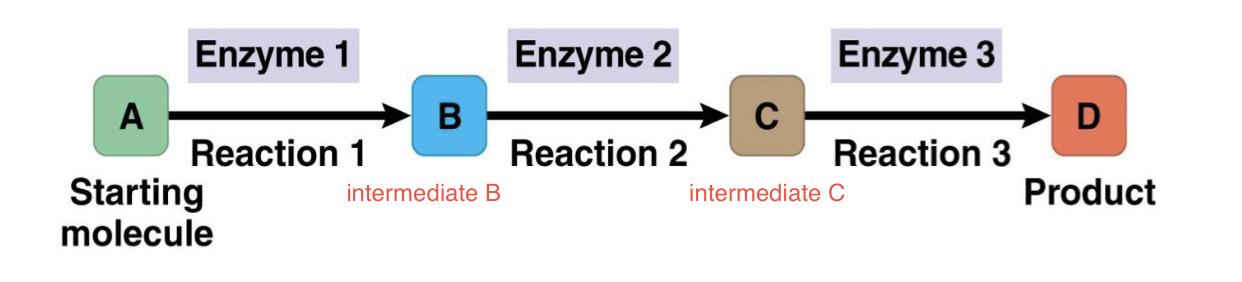
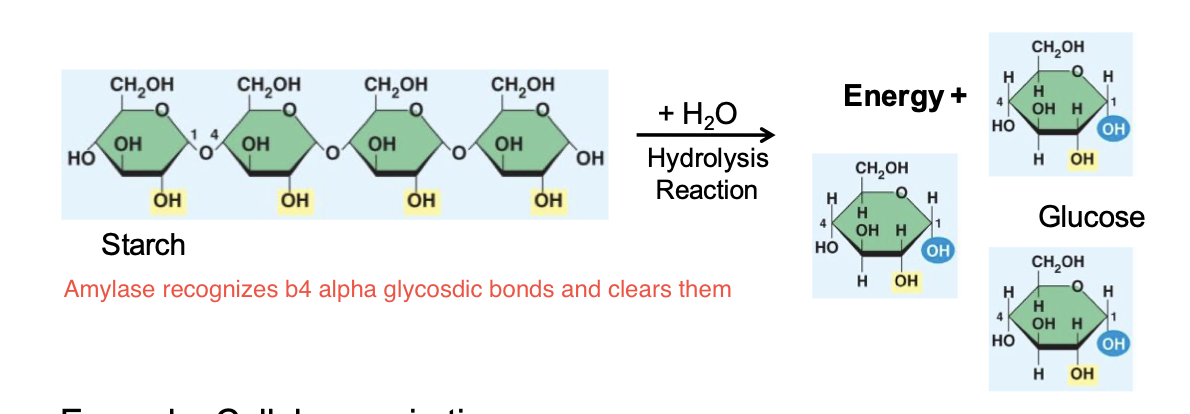
Catabolic Pathway
Series of chemical reactions that break down complex molecules into simpler molecules
→Releases energy = exergonic
→Requires enzymes
What are two examples of a catabolic pathway?
Breakdown of starch into glucose and Cellular respiration
Anabolic Pathways
Definition: Series of chemical reactions that
build complex molecules from simpler molecules
▪ Requires energy = endergonic
▪ Requires enzymes
What’s are two examples of anabolic pathways?
Synthesis of polypeptide from amino acid monomers
-Dehydration reaction
And photosynthesis
Energy coupling
Energy released from one reaction is used to power another reaction that requires energy
Energy released from carbolic (exergonic) reactions used to power anabolic (endergonic) reactions
Why is ATP important?
Powers cellular work by coupling exergonic/catabolic processes to endergonic/anabolic processes
What are the 3 cellular work that relies on ATP?
1. Movement:
→Movement of transport vesicles around cell
→Flagella and cilia
2. Transport:
→Moves substances across membrane against concentration gradient
3. Chemical:
→Driving endergonic chemical reactions
What is the structure of ATP?
Three phosphate groups
Ribose sugar
Adenine nitrogenous base (purine, a monomer of a long polymer [nucleic acid])
Nucleotide
Energy is released when the terminal (end) phosphate bond is removed
Why is ATP hydrolysis exergonic?
Repulsive forces between negatively charged phosphate groups make ATP less stable than ADP
How does ATP perform cellular work?
ΔG = -7.3 kcal/mol → energy is available to drive endergonic processes
Energy is harnessed by phosphorylation
▪ Phosphate from ATP hydrolysis is added to another molecule
What does phosphorylation make molecules?
Phosphorylation makes molecule more reactive causing endergonic chemical reactions to occur
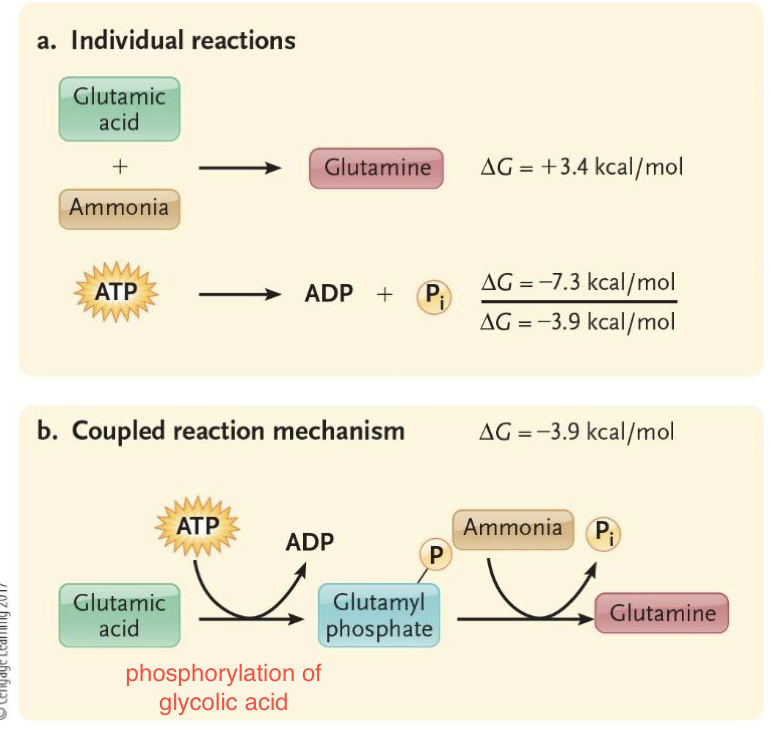
Phosphorylation can cause a protein to change shape
o Example: Transport proteins
Binding and hydrolyzing ATP can cause a protein to change shape
o Example: Motor proteins
What does the storing of covalent bonds depend on?
Electrons in covalent bonds store different amounts of energy depending on the electronegativity of of atoms involved
How does energy re
Electrons release energy going from a less electronegative atom to a more electronegative atom
Redox reaction
Chemical reaction where there is a transfer of electrons from one reactant to another
Oxidation
Loss of electrons (LEO)
Reduction
Gain of electrons (GER)
Cell stores in electron shuttle molecules
Electron shuttle molecules
Temporary storage site for high energy electrons
Ex: NAD+
Enzyme that removes 2 hydrogen atoms from high energy substrate and transfers them to shuttle molecules
A series of electron-carrying membrane proteins that transfer electrons and release energy
-Receive high energy electrons (from electron shuttles)
Energy is released
What is an enzyme?
Catalytic proton that speed up a reaction w/o being consumed itself
→ Increases reaction rate
→ Does not change the reaction
→ Does not provide energy to the reaction
→ Used in both exergonic and endergonic reactions
→ Activity can be regulated
How do enzymes impact a reaction without changing the overall delta G?
It lowers Ea
Reaction occurs at safe temp. for cells
Reaction occurs rapidly
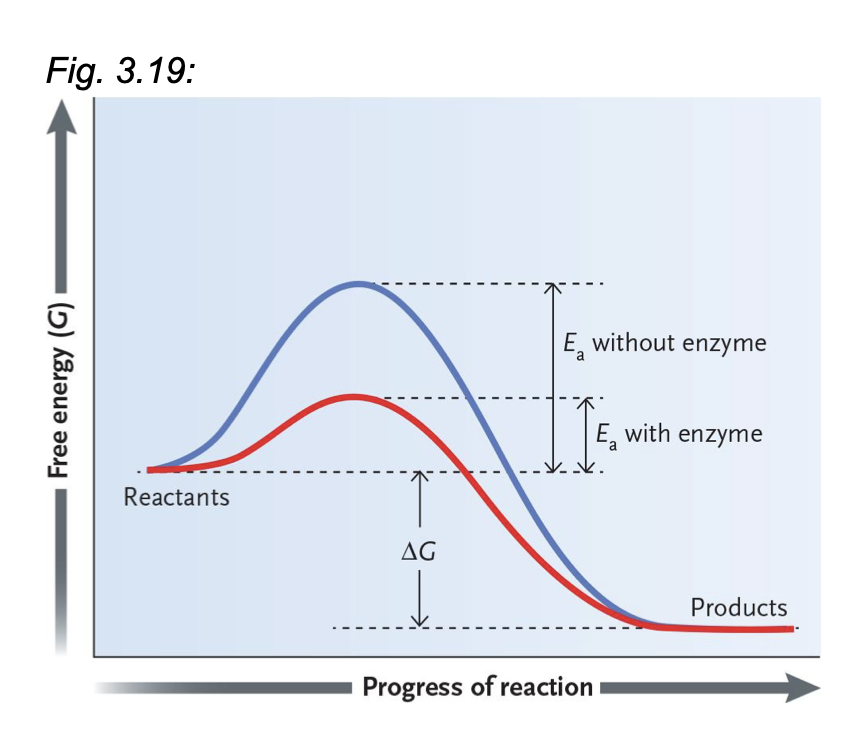
How does an enzyme work?
• Substrate: Reactant that an enzyme acts upon
• Product: Molecules produced at the end of the reaction
What is the active site in an enzyme?
→ Pocket on the surface of the enzyme where the substrate binds and where the reaction happens
→ Enzyme specific active site to its substrate due to of active site
▪ Shape due to the amino acid sequence of the protein
→ Amino acids in the active site specifically interact with the substrate
What is the 1st mechanism that is part of how an enzyme works?
Substrate enters active site
→ Enzyme changes shape to interact with substrate
→ Called induced fit
What is the 2nd mechanism that is part of how an enzyme works?
Substrate is held in place in the active site of the enzyme
→ H-bonds, hydrophobic interactions, charge interactions form between active site amino acids and substrate
What is the 3rd mechanism that is part of how an enzyme works?
Activation energy is lowered by the active site (3 ways this can be done)
a) Enzyme lines up the substrates correctly for new bonds to form
b) Bonds in the substrate are stressed, making them easier to break
c) Creates a charge environment that favours the reaction
What is the 4th mechanism that is part of how an enzyme works?
Substrate is converted into products that are released from active site
→ Products have a different shape than substrates and don’t fit in active site
Cycle repeats
What determines enzyme activity?
Concentration of substrate
→ More substrate = faster reaction rate
→ Rate increases until a active sites in all enzymes are full = maximum rate
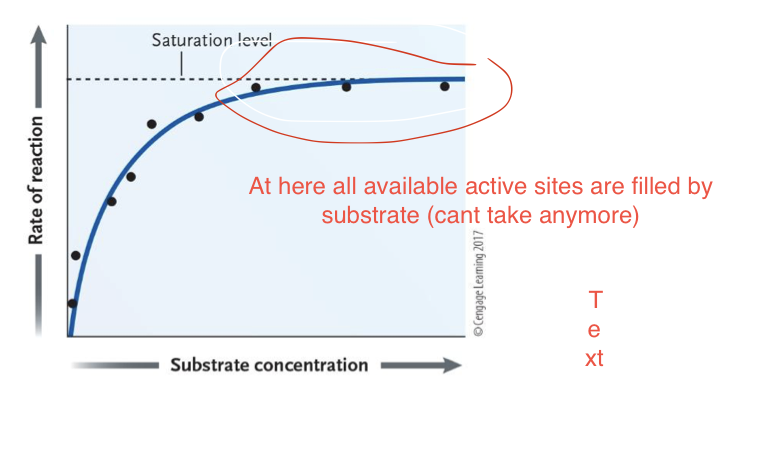
What does more enzyme in concentration mean for the reaction?
→ More enzyme = faster reaction rate
As long as there is enough substrate for all enzymes
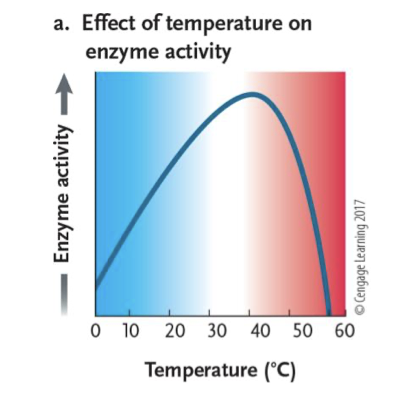
What happens with temperature when an enzyme is used in a reaction?
↑ temperature = ↑ enzyme/substrate interactions = ↑ reaction rate
→ Too much heat = enzymes denature = lower reaction rate
→ Optimal temperature produces
fastest rate (refer to graph)
What happens with pH when an enzyme is used in a reaction?
Affects charges on amino acid side chains
→ Changes in pH = changes protein shape and ability of substrate to bind in active site
= decreased reaction rate
→ Optimal pH produces fastest rate
Cofactors and Coenzymes
Cofactors = metal ions or inorganic minerals
Coenzymes = organic molecules (vitamins)
Non-protein helpers that are required for catalytic activity of enzymes
Enzyme Inhibitors
Chemicals that selectively decrease activity of an enzyme
May be:
chemical, drugs, poisons
normal molecules in the cell
Two types of inhibitors?
1) Irreversibile Inhibitor
-Bind to enzyme using covalent bond
-Permanent inactivation
2) Reversible Inhibitor
-Binds to enzymes using hydrogen bonds
-Temporary inactivation (enzyme will return to normal when inhibitor is removed)
-Two types
A) Competitive Inhibitor
Competes w/ substrate for binding to active site
Inhibitor block the active site & prevents formation of enzyme-substrate complex = decreased reaction rate
Can be overcome by increasing substrate concentration
B) Non-Competitive Inhibitor
Bind to region of enzyme other than active site
Changes the shape of the enzyme to make it less able to bind substrate
Does NOT compete with substrate for active site
CANNOT be overcome by adding more substrate