IU BIO-L 112 Chooi Exam 3
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
What is immunology?
The study of how the body responds to and resists foreign pathogens and other foreign substances.
What are pathogens?
Microorganisms such as bacteria and viruses that can cause disease.
What is variolation?
Deliberate infection with smallpox, historically used to reduce mortality rates.
Who introduced variolation to European aristocracy?
Lady Montague, who variolated her children in 1721.

What was the fatality rate of variolation compared to unvariolated smallpox?
1% for variolated individuals versus 30% for unvariolated.
What does vaccination derive from?
The Latin word 'vaca,' meaning cow.
Who was Benjamin Jetsy and what did he discover?
In 1774, he inoculated his family with cowpox, which prevented smallpox.
What significant experiment did Edward Jenner conduct in 1796?
He inoculated James Phipps with cowpox and then variolated him with smallpox, resulting in no smallpox infection.
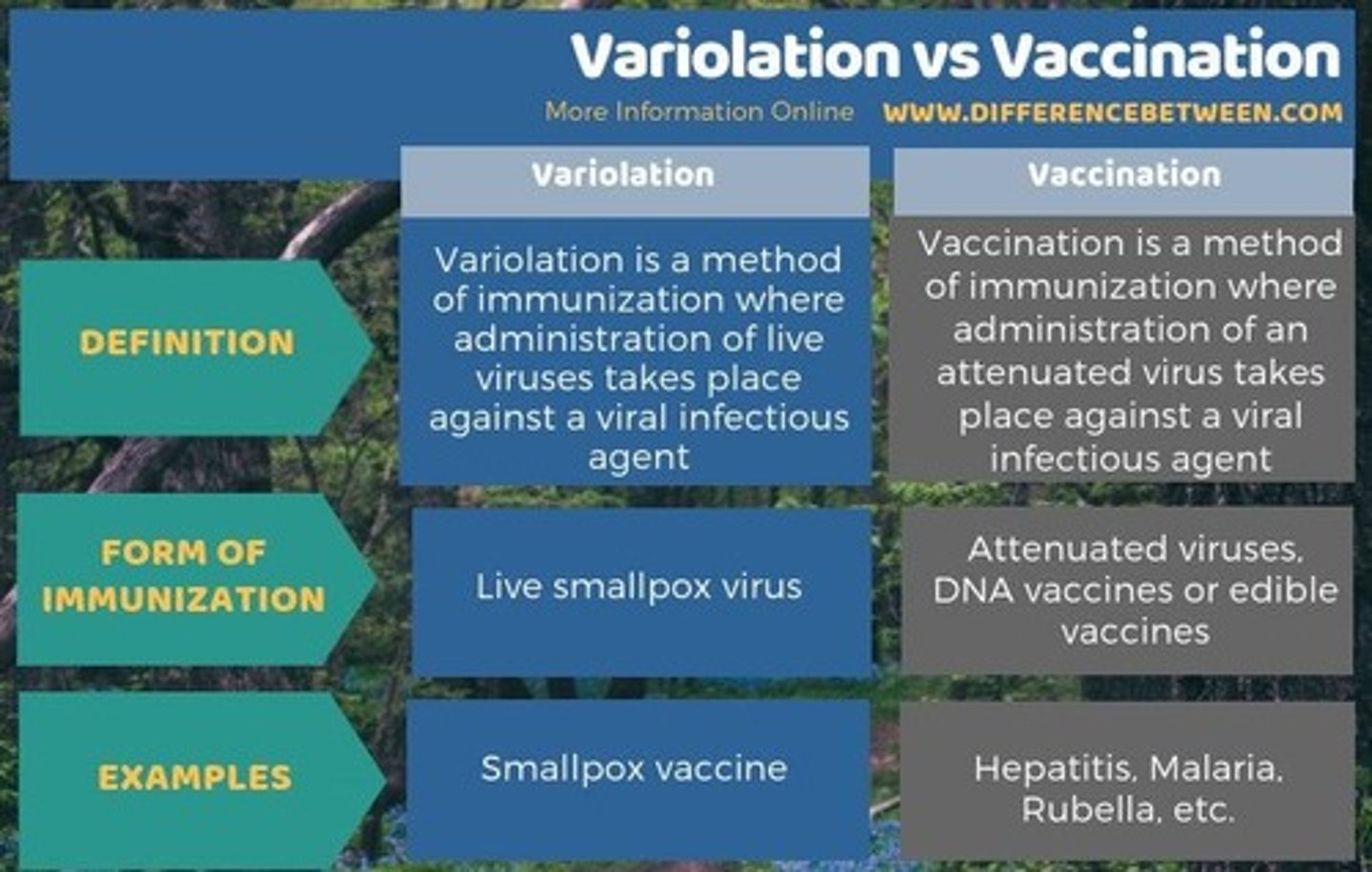
What are the main differences between variolation and vaccination?
Variolation had variable success and risks, including fatalities and infectiousness, while vaccination is more controlled.
What does attenuation mean in the context of immunology?
To weaken a viable pathogen to create a vaccine.
Who is associated with the concept of attenuation?
Louis Pasteur, who discovered that old cultures of pathogens could protect against re-challenge.

What methods are used to achieve attenuation?
Heat kill, serial passage in eggs, passage in cell culture, and genetic engineering.
What are the two types of immune defenses?
Adaptive defenses (activated by specific pathogens) and innate defenses (always deployed).
What are examples of external innate defenses?
Skin and mucous membranes.
What are examples of internal innate defenses?
Phagocytic cells, lymphocytes, and defensive proteins.
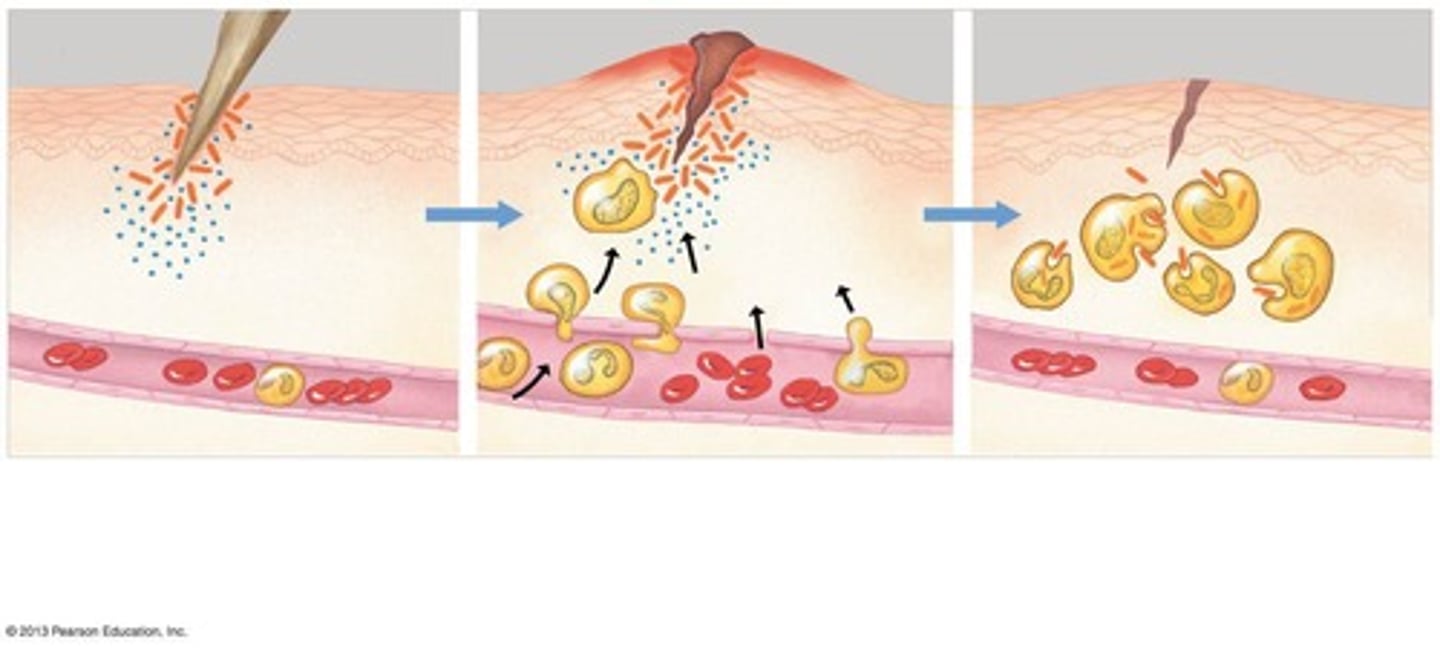
What is the inflammatory response?
An innate internal defense mechanism involving blood clotting, swelling, and the migration of phagocytic cells.
What are the two types of lymphocytes involved in adaptive defenses?
B cells (humoral immunity) and T cells (cell-mediated immunity).
Where do B cells mature?
In the bone marrow.
Where do T cells mature?
In the thymus.
What are antigens?
Molecules on the surfaces of viruses or foreign cells that elicit a response from lymphocytes.
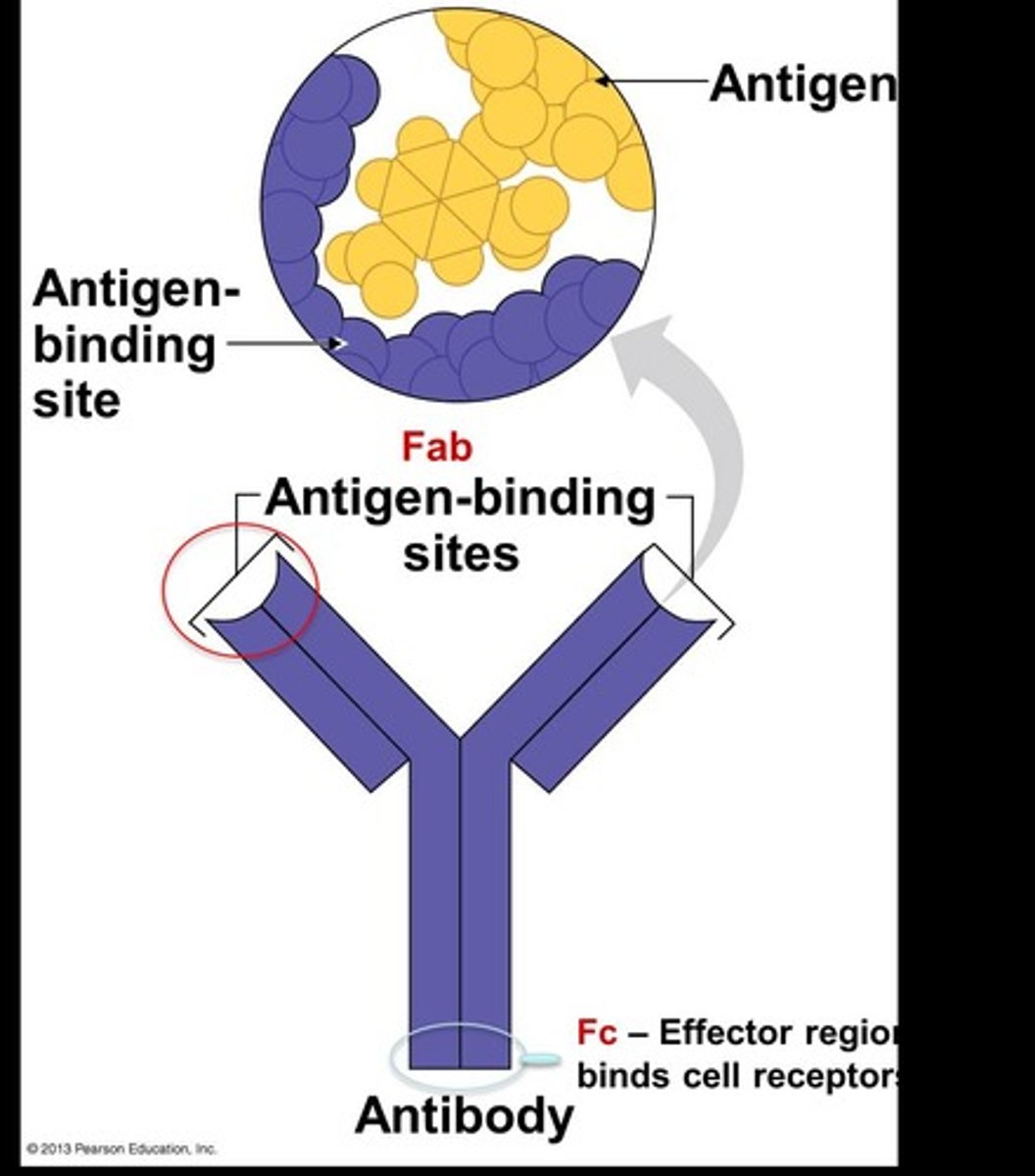
What are the five classes of immunoglobulins (Ig)?
IgM, IgG, IgA, IgE, IgD.
What is the role of B cells in the immune response?
They secrete free-floating antibodies into the blood and lymph.
What is the role of T cells in the immune response?
They are involved in cell-mediated immunity and can differentiate into various types (TH, Tc, Td, Ts, Treg).
What are the five classes of antibodies?
IgM, IgD, IgG, IgA, IgE.
What is clonal selection in the immune response?
The process of multiplying lymphocytes in response to an antigen, producing effector cells and memory cells.

What are the two types of cells produced by clonal selection?
Effector cells (Plasma cells) that produce antibodies and memory cells that are long-lived.
How long does the primary immune response take to produce effector cells?
7-14 days.
What is the role of memory cells in the immune system?
They respond to subsequent exposures to previously encountered antigens and can last for decades.
What is the lymphatic system?
A network of vessels, tissues, and organs that helps remove toxins, waste, and pathogens, and facilitates immune responses.
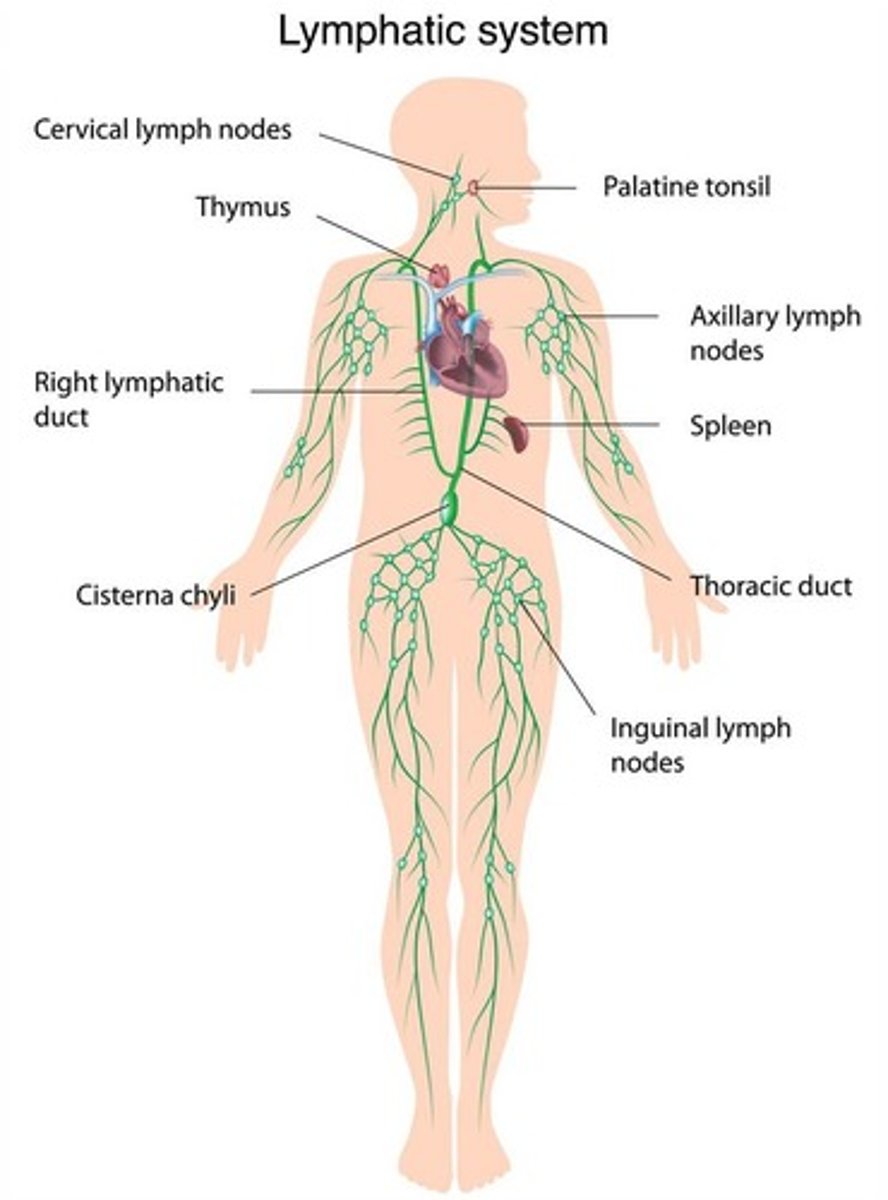
What are the main functions of the lymphatic system?
Drains interstitial fluid, maintains fluid balance, transports fatty acids, and transports white blood cells.
What are the three lines of defense in the immune system?
Innate immunity, adaptive immunity, and immunological memory.
What characterizes innate immunity?
It is fully ready to respond before an invader is encountered, including physical barriers and internal defenses.
What are some examples of innate immunity defenses?
External barriers like skin, secretions, phagocytic cells, natural killer cells, and defensive proteins.
What is adaptive immunity?
Activated by exposure to specific invaders, involving lymphocytes (B cells and T cells).
What is the difference between the primary and secondary immune responses?
The secondary immune response is faster and stronger due to the presence of memory cells.
What is a type I hypersensitivity reaction?
An allergic reaction mediated by IgE, often occurring on mucosal surfaces.
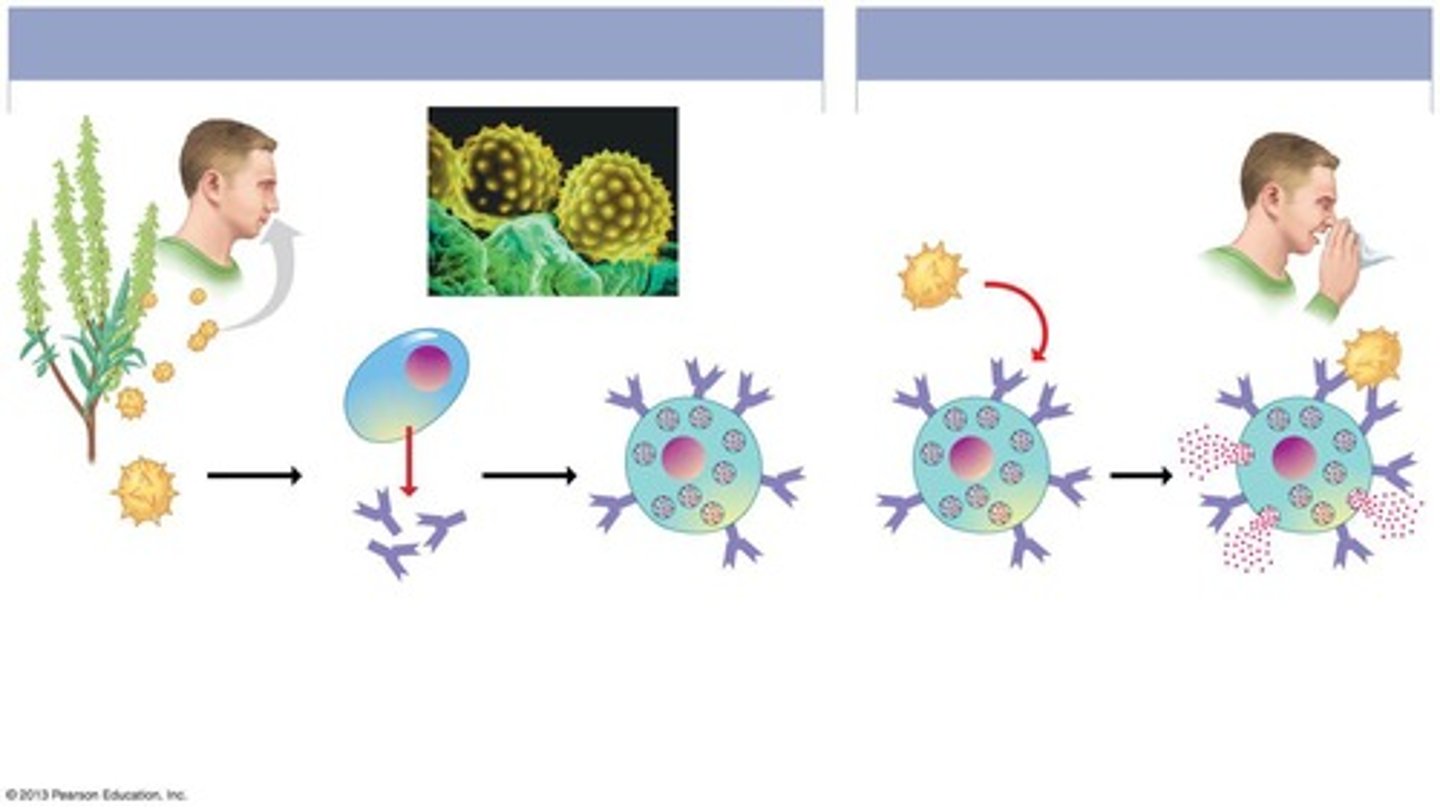
What is an allergen?
A substance that induces a type I hypersensitive reaction, often unclear what specifically qualifies as one.
What role do IgE antibodies play in allergies?
They bind to Fc receptors on basophils and mast cells, leading to allergic symptoms upon re-exposure to the allergen.
What is sensitization in the context of allergies?
The initial exposure to an allergen that primes the immune system for future reactions.
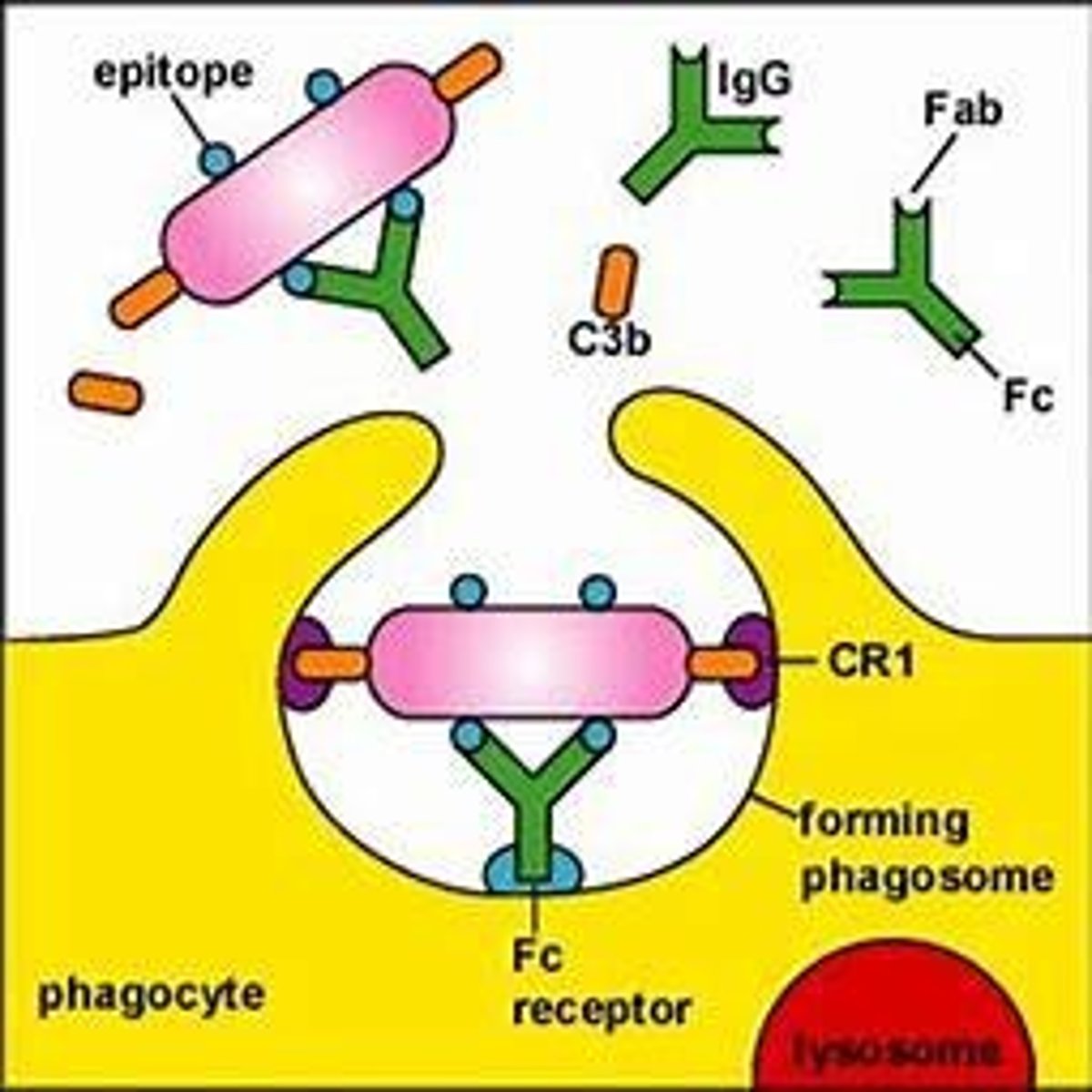
What is phagocytosis?
The process by which phagocytes engulf and digest pathogens and other molecules.
What are the symptoms of an allergic response?
Runny nose, headaches, rash, breathing trouble, nausea, diarrhea, and anaphylactic shock.
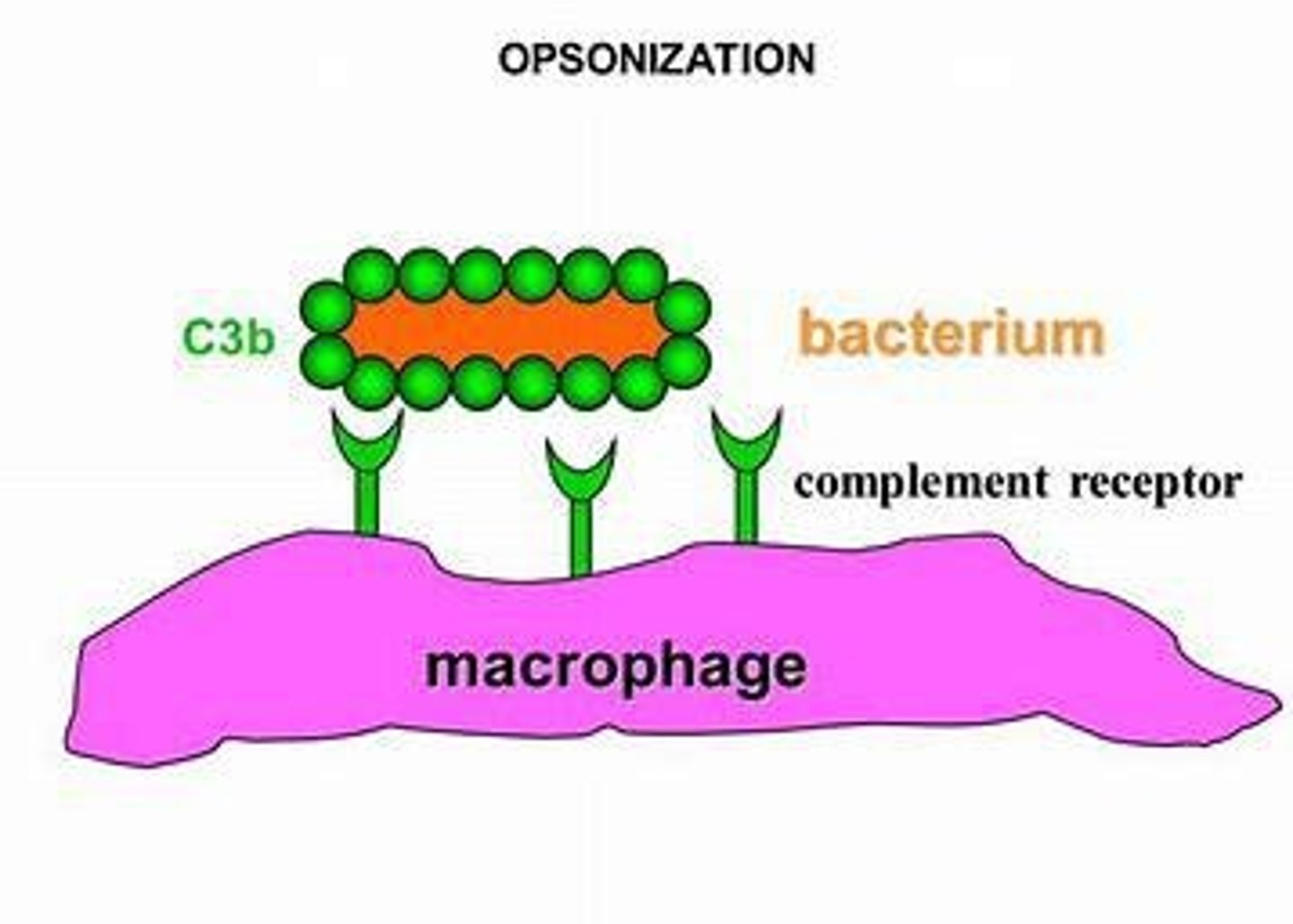
What is opsonization?
The process that enhances phagocytosis by marking pathogens for destruction.
What is the role of histamine in allergic reactions?
Histamine is released by mast cells and basophils, causing allergy symptoms.
What is the significance of TH2-like cytokines in allergies?
They activate antibody responses to parasites, bacteria, and allergens, heavily associated with allergic reactions.
What is the primary immune response?
The body's first response to exposure to an antigen, characterized by the production of antibodies.
What is the function of effector B cells?
They produce antibodies in response to an antigen.
What is opsonization?
A process that enhances phagocytosis by tagging pathogens, making them easier to engulf.
What role does complement C3b play in opsonization?
C3b coats the pathogen and enhances phagocytosis.
What is an opsonin?
A molecule that enhances phagocytosis, such as IgG, which fixes complement.
During which phase of the cell cycle does DNA replication occur?
S phase.
Who was the first person to produce an image of DNA using X-ray crystallography?
Rosalind Franklin.
What structural model did Watson and Crick propose for DNA?
A double helix with two outer sugar/phosphate backbones and paired bases in the interior.
What is the significance of the antiparallel nature of DNA backbones?
The two backbones run in opposite directions, which is essential for replication and function.
Which bases are classified as purines?
Adenine (A) and Guanine (G).
Which bases are classified as pyrimidines?
Cytosine (C), Uracil (U), and Thymine (T).
What is the base pairing rule for DNA?
A pairs with T (2 hydrogen bonds) and C pairs with G (3 hydrogen bonds).
What is the semiconservative model of DNA replication?
Each daughter molecule consists of one old strand and one new strand.
How many origins of replication do prokaryotic cells have?
A single origin of replication.
How do eukaryotic cells differ in their origins of replication compared to prokaryotic cells?
Eukaryotic cells can have many origins of replication that open into replication bubbles.
What is the role of primase in DNA replication?
Primase lays down a short RNA primer that is complementary to the parental DNA strand.
What direction does DNA polymerase synthesize the leading strand?
In the 5' to 3' direction.
What are Okazaki fragments?
Segments of DNA synthesized on the lagging strand, joined together by DNA ligase.
What is the function of DNA polymerase during DNA replication?
It synthesizes new DNA strands and proofreads for errors.
What is mismatch repair?
A process where repair enzymes correct base pair errors in DNA.
What is nucleotide excision repair?
A mechanism where nucleases cut out and replace damaged stretches of DNA.
What is the central dogma of molecular biology?
The process of DNA being transcribed to RNA, which is then translated into protein.
How do prokaryotic and eukaryotic transcription differ?
In prokaryotes, translation can begin before transcription finishes; in eukaryotes, they are separated by the nuclear envelope.
What happens to eukaryotic RNA transcripts before translation?
They undergo RNA processing to yield the finished mRNA.
What is the template strand in transcription?
The DNA strand that provides a template for ordering the sequence of complementary nucleotides in an RNA transcript.
What is the process by which eukaryotic RNA transcripts are modified to yield finished mRNA?
RNA processing.
What is the role of the template strand during transcription?
It provides a template for ordering the sequence of complementary nucleotides in an RNA transcript.
In which direction are mRNA codons read during translation?
In the 5′ → 3′ direction.
What do codons in mRNA specify?
Each codon specifies one of the 20 amino acids to be placed at the corresponding position along a polypeptide.
What are the two main modifications made to pre-mRNA during RNA processing?
Both ends of the primary transcript are altered, and introns are cut out while exons are spliced together.
What are spliceosomes composed of?
A variety of proteins and several small nuclear ribonucleoproteins (snRNPs) that recognize splice sites.
What is the function of the 5' cap added during RNA processing?
It helps protect the mRNA from degradation and assists in ribosome binding during translation.
What is the purpose of the poly-A tail added to the 3' end of mRNA?
It aids in the stability and export of mRNA from the nucleus and enhances translation.
What are the three sites of a ribosome during translation?
P site (Peptidyl-tRNA binding site), A site (Aminoacyl-tRNA binding site), and E site (Exit site).
What occurs during the initiation phase of translation?
The small ribosomal subunit binds to mRNA and moves along it until it reaches the start codon (AUG).
What are the three steps of elongation in translation?
Codon recognition, peptide bond formation, and translocation.
What triggers termination in translation?
A stop codon in the mRNA reaches the A site of the ribosome.
What is the role of the release factor during termination?
It promotes hydrolysis, causing the polypeptide to be released and the ribosomal subunits to dissociate.
What are insertions and deletions in the context of genetic mutations?
They are additions or losses of nucleotide pairs in a gene that can lead to frameshift mutations.
How do insertions and deletions affect protein synthesis compared to substitutions?
They often have a more disastrous effect on the resulting protein than substitutions do.
What is a frameshift mutation?
A mutation that alters the reading frame of the genetic code due to insertions or deletions.
What is a silent mutation?
A nucleotide-pair substitution that does not change the amino acid sequence of the resulting protein.
What is a missense mutation?
A nucleotide-pair substitution that results in a change in the amino acid sequence of the protein.
What is the role of RNA polymerase during transcription?
It synthesizes RNA by unwinding the DNA and adding complementary RNA nucleotides.
What happens to the DNA after RNA polymerase transcribes a gene?
The DNA rewinds back into its double helix structure.
What is the significance of the polyadenylation signal in mRNA processing?
It signals the addition of the poly-A tail to the 3' end of the mRNA.
What is the function of the untranslated regions (UTRs) in mRNA?
They play roles in the regulation of translation and stability of the mRNA.
What is the start codon and its significance?
The start codon (AUG) signals the beginning of translation and codes for the amino acid methionine.
What are the stop codons and their role in translation?
Stop codons (UAG, UAA, UGA) signal the termination of translation.